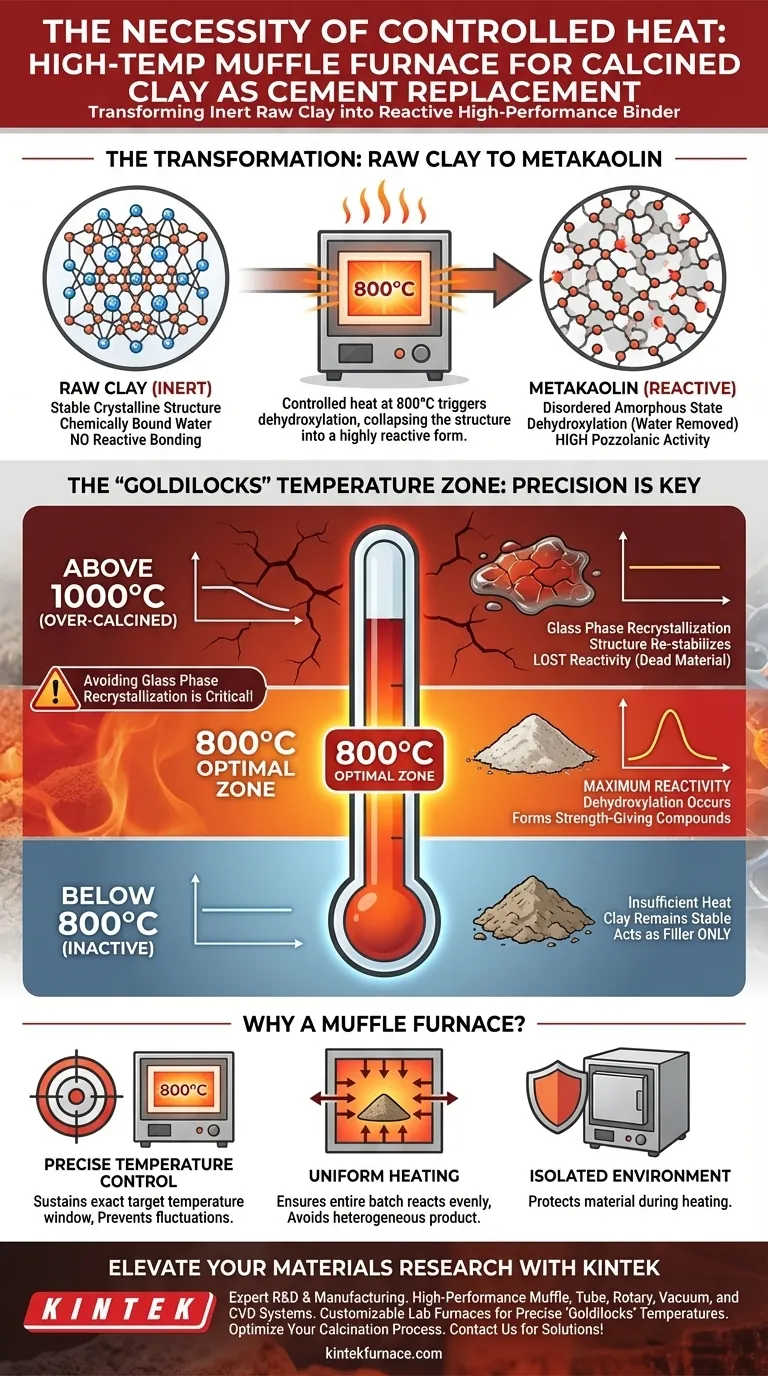
Controlled heat treatment is the distinct mechanism that transforms inert raw clay into a reactive, high-performance cement substitute. A high-temperature muffle furnace is necessary to maintain a precise environment—typically around 800 degrees Celsius—which triggers dehydroxylation in the clay lattice to produce metakaolin with high pozzolanic activity.
The Core Insight Natural clay is structurally stable and unreactive; to become a viable cement replacement, it must be thermally destabilized. The muffle furnace provides the exact thermal energy required to strip water from the mineral structure without overheating it, ensuring the material remains reactive rather than turning into inert glass.

The Physics of Activation
Transforming Structure Through Heat
Raw, natural clay does not naturally bond with concrete components. To make it useful, you must alter its chemical structure through a process called calcination.
A high-temperature muffle furnace allows you to elevate the clay to approximately 800 degrees Celsius. This specific thermal environment is critical for converting the base clay into metakaolin.
The Mechanism of Dehydroxylation
The primary goal of this heat treatment is dehydroxylation.
This involves removing the hydroxyl groups (chemically bound water) from the clay mineral lattice. When these groups are driven off by the heat, the crystal structure collapses into a disordered, amorphous state. This disordered state is highly reactive, or "pozzolanic."
Ensuring Pozzolanic Activity
Pozzolanic activity is the measure of how well the clay will react with calcium hydroxide in cement to form strength-giving compounds.
Without the precise heat application provided by the furnace, the clay retains its original, stable structure. Consequently, it would act merely as a filler rather than an active binding agent.
The Critical Importance of Precision
The "Goldilocks" Temperature Zone
Achieving high reactivity is not simply about getting the material hot; it is about hitting a specific temperature window.
The muffle furnace provides the control necessary to sustain the target temperature (e.g., 800°C). This consistency ensures the reaction penetrates the entire batch evenly.
Avoiding Glass Phase Recrystallization
There is a distinct upper limit to beneficial heat treatment.
If the temperature spikes too high or fluctuates upward, the clay minerals can undergo glass phase recrystallization. This phenomenon organizes the structure back into a stable, non-reactive form.
Once recrystallization occurs, the material loses its ability to react with cement. The muffle furnace prevents this by capping the temperature, ensuring dehydroxylation occurs without crossing the threshold into recrystallization.
Understanding the Trade-offs
The Risk of Over-Calcination
While insufficient heat leaves clay inactive, excessive heat destroys its potential.
Pushing temperatures beyond the optimal 800°C range (approaching 1000°C or higher, as might be used for other ceramic composites) can lead to the formation of stable phases that are effectively "dead" in a cementitious context. You must avoid the temptation to overheat in an attempt to speed up the process.
Equipment Limitations
Standard ovens often lack the insulation and heating element power to maintain 800°C uniformly.
Using equipment unable to hold this "soak" temperature results in a heterogeneous product—parts of the clay may be burnt (inert) while others remain raw (inactive).
Making the Right Choice for Your Goal
To maximize the efficacy of calcined clay in your cement mix, consider these factors:
- If your primary focus is Strength Development: Prioritize strict temperature adherence at 800°C to maximize the formation of reactive metakaolin.
- If your primary focus is Consistency: Ensure your muffle furnace is calibrated to prevent temperature overshoots that cause recrystallization.
Precision in thermal treatment is the only variable standing between a high-performance binder and a pile of inert dirt.
Summary Table:
| Stage | Temperature Range | Structural Effect | Resulting Material Property |
|---|---|---|---|
| Raw State | Ambient | Stable crystalline lattice | Inert filler; no reactive bonding |
| Calcination | ~800°C | Dehydroxylation (disordered state) | High pozzolanic activity; reactive metakaolin |
| Over-heating | >1000°C | Glass phase recrystallization | Chemically stable; lost reactivity |
| Improper Heating | Fluctuating | Heterogeneous batch | Inconsistent strength and quality |
Elevate Your Materials Research with KINTEK
Precision is the difference between a reactive binder and inert waste. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of calcined clay production and cementitious research. Our lab high-temp furnaces are fully customizable to ensure your materials reach the exact 'Goldilocks' temperature every time.
Ready to optimize your calcination process? Contact our technical experts today to find the perfect furnace solution for your unique laboratory needs!
Visual Guide
References
- Marko Ćećez, Marijana Serdar. Autogenous shrinkage of cementitious composites incorporating red mud. DOI: 10.1515/rams-2025-0136
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the benefits of using a high-temperature sintering furnace at 350°C for PEEK? Maximize Composite Performance
- What role do muffle furnaces play in pharmaceutical research and development? Essential for Quality Control and Innovation
- How does a vacuum tube furnace differ from a vacuum muffle furnace? Choose the Right Furnace for Your Lab
- What are the advantages of using a box type resistance furnace? Achieve Precision Heating for Your Lab
- What is the conclusion regarding the comparison between muffle furnaces and vacuum furnaces? Choose the Right Furnace for Your Process
- What is a key feature of box furnaces regarding temperature control? Achieve Precise and Uniform Heating for Your Lab
- What are the common uses of a muffle furnace in material testing? Essential for Precise Thermal Analysis and Sample Prep
- What is the function of a high-temperature muffle furnace? Master Polycrystalline MgSiO3 and Mg2SiO4 Synthesis



















