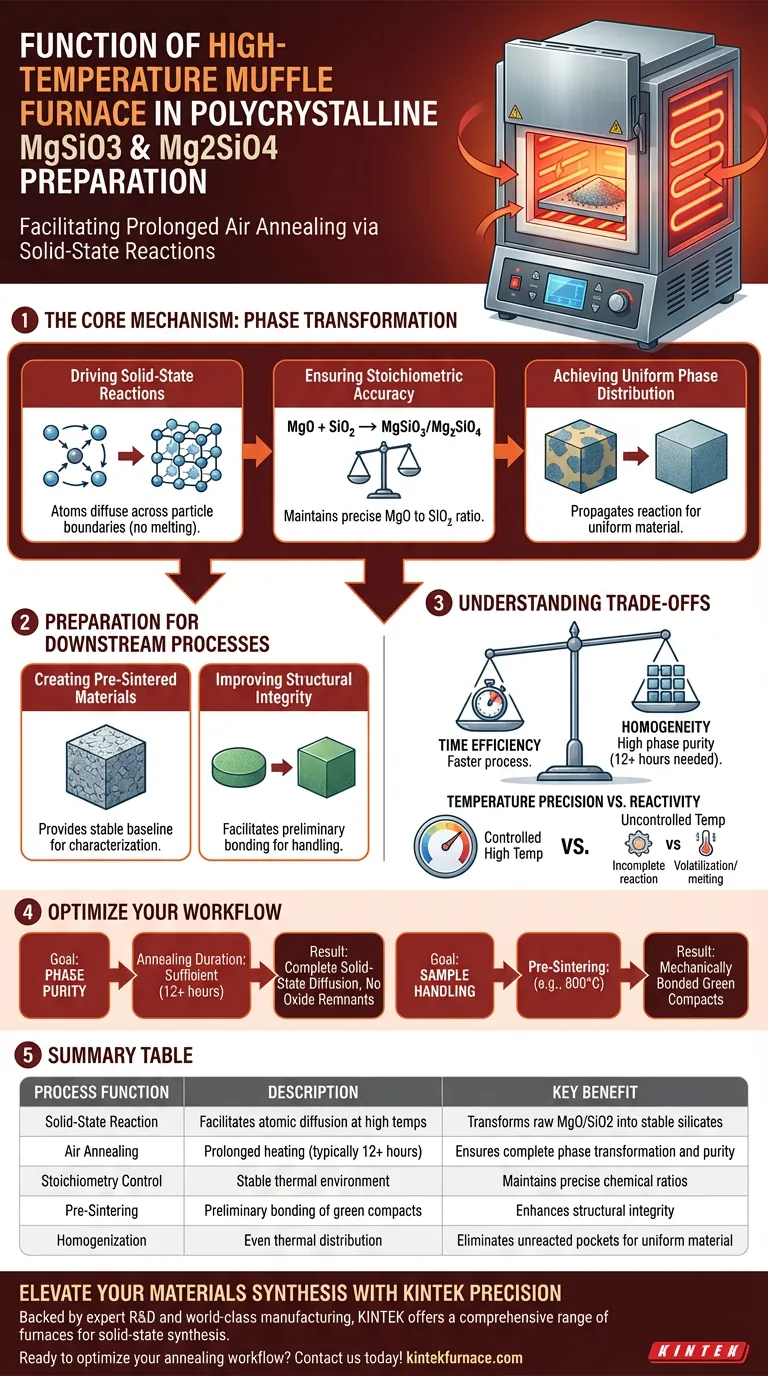
The primary function of a high-temperature muffle furnace in this context is to facilitate prolonged air annealing via solid-state reactions. By subjecting stoichiometric mixtures of Magnesium Oxide (MgO) and Silicon Dioxide (SiO2) to sustained heat—typically for durations around 12 hours—the furnace converts raw powders into stable polycrystalline magnesium silicate phases.
Core Takeaway The muffle furnace serves as a precision reactor that transforms raw chemical mixtures into uniform, pre-sintered materials. Its specific role is to drive solid-state diffusion to achieve accurate chemical compositions and phase distribution, ensuring the material is ready for complex downstream applications like high-pressure experiments.

The Mechanism of Phase Transformation
Driving Solid-State Reactions
The preparation of polycrystalline MgSiO3 (Enstatite) and Mg2SiO4 (Forsterite) relies on solid-state reactions.
Unlike processes that melt materials, this technique keeps the mixture in a solid phase. The muffle furnace provides the necessary thermal energy to overcome activation barriers, allowing atoms to diffuse across particle boundaries and form new crystal structures.
Ensuring Stoichiometric Accuracy
Achieving the correct chemical balance is critical for these silicates.
The furnace creates a stable, high-temperature environment that maintains the stoichiometry of the initial mixture. This ensures that the final product corresponds exactly to the intended ratio of MgO to SiO2, preventing the formation of unwanted secondary phases.
Achieving Uniform Phase Distribution
Raw mixtures often contain pockets of unreacted material.
Through prolonged annealing (e.g., 12 hours), the furnace ensures the reaction propagates through the entire bulk of the sample. This results in a homogeneous material where the target phase is distributed uniformly throughout the volume.
Preparation for Downstream Processes
Creating Pre-Sintered Materials
The output of this furnace stage is generally categorized as "pre-sintered" material.
This intermediate state is essential for researchers. It provides a stable baseline material that can be characterized or processed further without the variability associated with raw powder mixtures.
Improving Structural Integrity
In many workflows, the raw powders are first formed into "green compacts" (cold-pressed shapes).
Heating these compacts in the muffle furnace facilitates preliminary bonding between powder particles. This improves the structural integrity of the sample, making it robust enough to withstand handling and subsequent rigorous processes, such as high-pressure hot re-pressing.
Understanding the Trade-offs
Time Efficiency vs. Homogeneity
Solid-state diffusion is inherently slow compared to liquid-phase reactions.
The trade-off for achieving high phase purity without melting is time; the furnace must maintain high temperatures for extended periods (12+ hours). Shortcuts in annealing time often result in incomplete reactions and residual raw oxides.
Temperature Precision vs. Reactivity
The furnace temperature must be strictly controlled.
If the temperature is too low, the diffusion rate is insufficient to form the polycrystalline phase. Conversely, if the temperature is uncontrolled and exceeds the melting point, the stoichiometry may be altered due to volatilization, or the microstructure may change drastically, defeating the purpose of solid-state synthesis.
Making the Right Choice for Your Goal
To maximize the utility of the muffle furnace in your synthesis workflow, consider your immediate experimental needs:
- If your primary focus is Phase Purity: Ensure the annealing duration is sufficient (typically 12 hours) to allow for complete solid-state diffusion and the elimination of raw oxide remnants.
- If your primary focus is Sample Handling: Utilize the furnace for a pre-sintering step (e.g., at 800°C) to mechanically bond green compacts before subjecting them to densification or high-pressure environments.
Ultimately, the muffle furnace acts not just as a heater, but as a standardization tool that guarantees the chemical fidelity of your starting materials.
Summary Table:
| Process Function | Description | Key Benefit |
|---|---|---|
| Solid-State Reaction | Facilitates atomic diffusion at high temps | Transforms raw MgO/SiO2 into stable silicates |
| Air Annealing | Prolonged heating (typically 12+ hours) | Ensures complete phase transformation and purity |
| Stoichiometry Control | Stable thermal environment | Maintains precise chemical ratios of the mixture |
| Pre-Sintering | Preliminary bonding of green compacts | Enhances structural integrity for high-pressure use |
| Homogenization | Even thermal distribution | Eliminates unreacted pockets for uniform material |
Elevate Your Materials Synthesis with KINTEK Precision
Don't let incomplete reactions or non-uniform phases compromise your research. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of solid-state synthesis.
Our lab high-temperature furnaces provide the thermal stability and precision required to achieve perfect stoichiometry in MgSiO3 and Mg2SiO4 preparation. Whether you need a standard setup or a fully customizable solution for unique high-pressure research, KINTEK delivers the reliability your lab deserves.
Ready to optimize your annealing workflow? Contact us today to find your perfect furnace solution!
Visual Guide
References
- Yuta Shuseki, Takehiko Ishikawa. Atomic and Electronic Structure in MgO–SiO<sub>2</sub>. DOI: 10.1021/acs.jpca.3c05561
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why are modern muffle furnaces considered energy-efficient? Discover Key Innovations for Lower Costs
- What is the primary function of a high-temperature muffle furnace in ilmenite smelting? Enhance Carbothermic Efficiency
- What are the main benefits of using a muffle furnace? Achieve Precise, Contamination-Free Heating for Your Lab
- How does a stainless steel reactor function within a muffle furnace for PET to graphene? Master Carbon Synthesis
- What makes box furnaces suitable for demanding applications? Engineered for Precision and Durability in High-Stakes Processes
- Why is a muffle furnace considered a sensitive product? Understand the High-Risk Hazards and Safety Needs
- What is the function of a muffle furnace in Li2Mg3Ti(1-x)ZrxO6 calcination? Optimize Ceramic Phase Purity
- What types of analyses can be performed using a muffle furnace in coal analysis? Unlock Key Coal Quality Insights



















