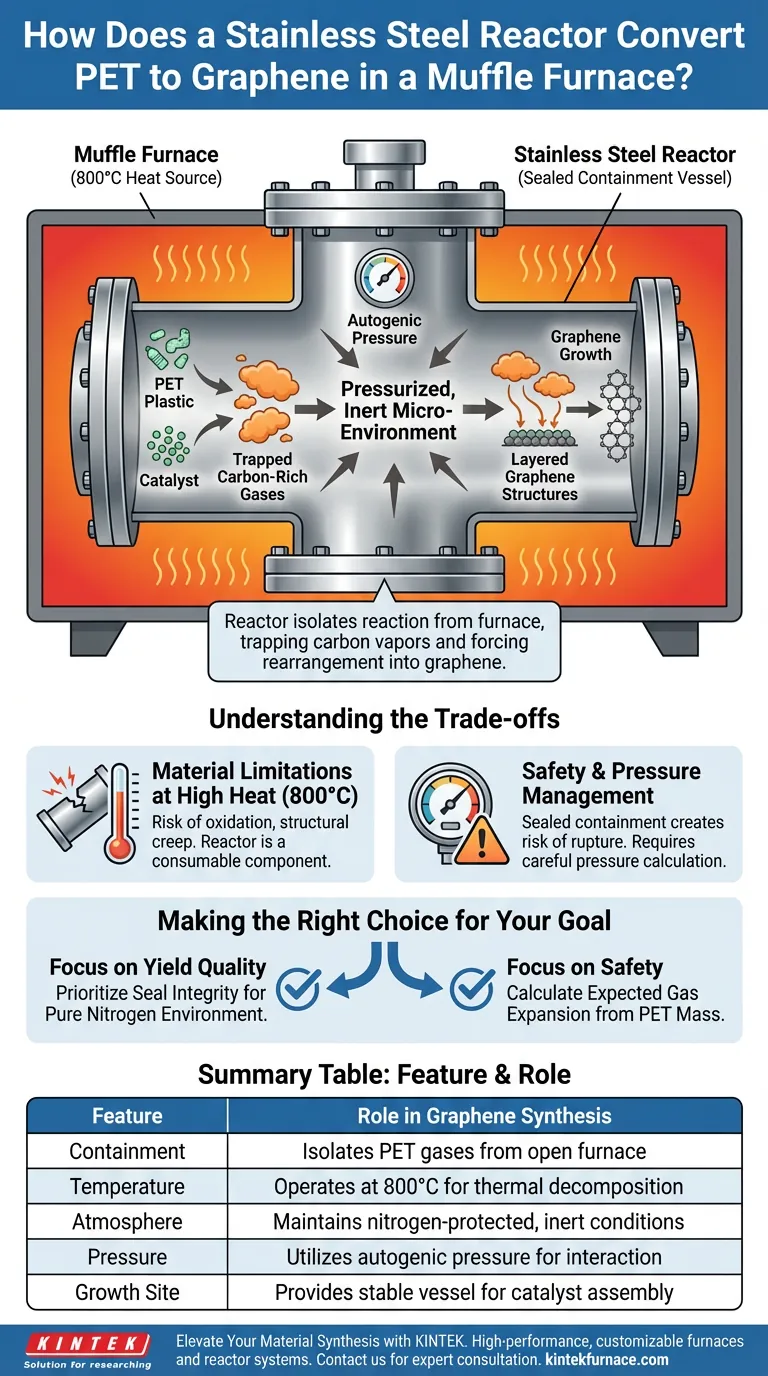
In this specific synthesis method, the stainless steel reactor acts as a hermetically sealed containment vessel that isolates the chemical reaction from the open furnace environment. It captures the carbon-rich gases released during the 800°C thermal decomposition of PET plastic, forcing them to interact with catalysts under high pressure rather than venting away.
The reactor transforms the open heat of a muffle furnace into a pressurized, inert micro-environment. This confinement is critical for trapping carbon vapors and forcing their rearrangement into layered graphene structures on a catalyst substrate.
The Mechanics of Confinement and Conversion
Creating a Controlled Micro-Environment
The primary function of the muffle furnace is simply to generate heat, reaching temperatures up to 800°C. However, the furnace chamber itself is often too large or chemically uncontrolled for precision synthesis.
The stainless steel reactor sits inside this heated zone, creating a distinct, smaller volume. This separation allows the internal environment to be nitrogen-protected, ensuring no oxygen enters to combust the plastic.
Trapping Carbon Gases
As the PET plastic undergoes thermal decomposition (pyrolysis), it releases volatile carbon-bearing gases. Without the reactor, these gases would dissipate into the furnace exhaust.
The sealed nature of the reactor traps these gases. This containment creates an environment rich in carbon feedstock necessary for graphene formation.
Generating Autogenic Pressure
Because the reactor is sealed, the release of gases from the decomposing plastic naturally increases the internal pressure.
This pressurized environment is not incidental; it works in conjunction with the high heat to drive the reaction kinetics. It forces the carbon atoms to interact more frequently with the catalyst surfaces.
Facilitating Graphene Growth
The Role of the Catalyst
The reactor does not work alone; it functions as a vessel for pre-placed catalysts.
The stainless steel walls hold the catalyst in the optimal zone where temperature and gas density are highest. This proximity ensures the carbon gases contact the catalyst to begin atomic rearrangement.
Structural Rearrangement
Inside this hot, pressurized vessel, carbon atoms dissociate from the polymer chains.
Under these specific conditions, the atoms reassemble. They grow into the characteristic layered graphene structures on the catalyst surface, a process that would fail in an open-air heating environment.
Understanding the Trade-offs
Material Limitations at High Heat
While stainless steel is robust, operating at 800°C pushes the material toward its thermal limits.
Repeated cycling at these temperatures can lead to oxidation of the reactor's exterior or structural creep over time. The reactor vessel must be viewed as a consumable component that may degrade after multiple synthesis runs.
Safety and Pressure Management
The very feature that makes this work—sealed containment—introduces risk.
Heating a sealed vessel creates significant internal pressure. If the reactor design does not account for the volume of gas generated by the specific mass of PET used, there is a risk of rupture or seal failure.
Making the Right Choice for Your Goal
To apply this synthesis method effectively, you must balance the benefits of confinement with the realities of high-temperature pressurized vessels.
- If your primary focus is yield quality: Prioritize the seal integrity of the reactor to ensure a pure nitrogen environment, as even trace oxygen will ruin the graphene.
- If your primary focus is safety: Calculate the expected gas expansion from your PET mass carefully to ensure the reactor's pressure rating is not exceeded at 800°C.
By strictly controlling the reactor's internal atmosphere, you turn waste plastic into high-value nanomaterials.
Summary Table:
| Feature | Role in Graphene Synthesis |
|---|---|
| Containment | Isolates PET pyrolysis gases from the open furnace atmosphere |
| Temperature | Operates at 800°C to drive thermal decomposition and rearrangement |
| Atmosphere | Maintains nitrogen-protected, inert conditions to prevent combustion |
| Pressure | Utilizes autogenic pressure to increase carbon-catalyst interaction |
| Growth Site | Provides a stable vessel for catalysts to facilitate atomic assembly |
Elevate Your Material Synthesis with KINTEK
Precision graphene production requires the perfect balance of heat and containment. KINTEK provides the high-performance thermal solutions you need to turn waste PET into advanced nanomaterials.
Backed by expert R&D and manufacturing, we offer high-quality Muffle Furnaces, Tube Furnaces, and Vacuum systems, all of which are fully customizable to meet your specific pressure and temperature requirements. Whether you are scaling up synthesis or conducting specialized lab research, our equipment ensures consistent results and maximum safety.
Ready to optimize your carbon conversion? Contact us today to consult with our experts on the ideal furnace and reactor setup for your unique needs.
Visual Guide
References
- Eslam Salama, Hassan Shokry. Catalytic fabrication of graphene, carbon spheres, and carbon nanotubes from plastic waste. DOI: 10.1039/d3ra07370j
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a muffle furnace play in the OBD process for Ti-6Al-4V? Enhance Alloy Surface Hardening Precision
- What is the function of a high-temperature Muffle Furnace in the two-step heat treatment of PTFE-coated Nickel Foam?
- What materials can be processed in a muffle furnace? Explore Versatile High-Temp Solutions
- What role does a laboratory high-temperature muffle furnace play in converting calcified pollen into bioceramics?
- What temperature range can box furnaces reach? Achieve 1800°C for Precise Thermal Processing
- What is the function of a high-temperature box furnace in the annealing process of AA6061 aluminum alloy?
- What temperature range can a muffle furnace operate within? Unlock High Heat and Precision for Your Lab
- How do muffle furnaces contribute to the production of technical ceramics? Achieve High-Purity, Dense Ceramics with Precision



















