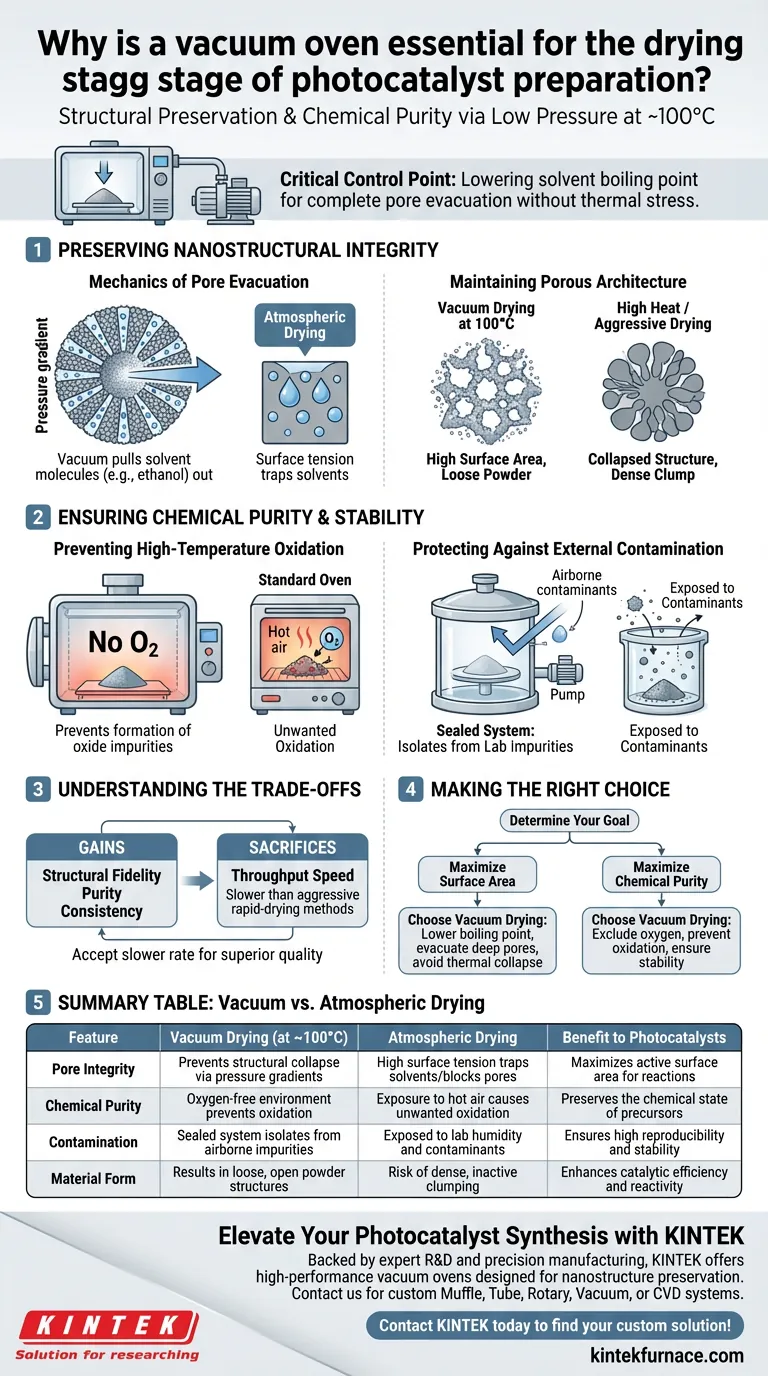
The use of a vacuum oven during photocatalyst preparation is not merely about speed; it is strictly a matter of structural preservation and chemical purity. By lowering the atmospheric pressure, the oven accelerates the removal of solvent molecules like ethanol from deep within nanostructure pores at manageable temperatures (around 100°C). This distinct environment prevents high-temperature oxidation and shields the material from external impurities, ensuring the final product maintains the specific physical properties required for reactivity.
The vacuum oven acts as a critical control point for defining surface area. By lowering the boiling point of solvents, it allows for complete pore evacuation without the thermal stress that causes structural collapse, ensuring the catalyst retains the maximum active surface area.

Preserving Nanostructural Integrity
The Mechanics of Pore Evacuation
The primary mechanical advantage of a vacuum oven is its ability to create a pressure gradient.
In standard atmospheric drying, surface tension can trap solvents deep within the intricate pore structures of the photocatalyst precipitate.
The vacuum environment effectively "pulls" these solvent molecules out, ensuring that the internal porosity is cleared and available for catalytic reactions.
Maintaining Porous Architecture
A highly developed pore structure is the engine of a photocatalyst.
If solvents are not fully removed, or if they are removed too aggressively via high heat, the pores can collapse or become blocked.
Vacuum drying at 100°C ensures the material creates a loose, open powder structure rather than a dense, inactive clump.
Ensuring Chemical Purity and Stability
Preventing High-Temperature Oxidation
Many photocatalyst precursors are sensitive to oxygen, particularly when heated.
Standard ovens expose the material to hot air, which can lead to unwanted oxidation of the active sites before the catalyst is even finished.
The vacuum chamber removes oxygen from the equation, preserving the chemical state of the precursors and preventing the formation of oxide impurities that dampen performance.
Protecting Against External Contamination
Catalyst preparation requires a controlled baseline to ensure reproducibility.
A vacuum oven operates as a sealed system, physically isolating the material from airborne contaminants and humidity found in the laboratory environment.
This isolation is critical for maintaining chemical stability and ensuring that the only reactions occurring are the ones you intended.
Understanding the Trade-offs
Drying Rate vs. Component Distribution
While vacuum drying is superior for pore preservation, it is not always the fastest method available.
Supplementary data suggests that vacuum drying rates can be lower than "quick drying" methods (such as convective rapid drying).
This slower rate can influence the distribution of active components, sometimes resulting in an intermediate "egg-shell" layer thickness.
You must accept that while you gain structural fidelity and purity, you may sacrifice the throughput speed offered by aggressive rapid-drying techniques.
Making the Right Choice for Your Goal
To determine if vacuum drying is the correct approach for your specific synthesis, consider your performance metrics:
- If your primary focus is maximizing surface area: Rely on vacuum drying to lower the solvent boiling point and evacuate deep pores without causing thermal collapse.
- If your primary focus is chemical purity: Use the vacuum environment to exclude oxygen, preventing the oxidation of sensitive precursors during the heating phase.
The vacuum oven transforms the drying process from a simple dehydration step into a fundamental quality assurance measure for high-efficiency catalysis.
Summary Table:
| Feature | Vacuum Drying (at ~100°C) | Atmospheric Drying | Benefit to Photocatalysts |
|---|---|---|---|
| Pore Integrity | Prevents structural collapse via pressure gradients | High surface tension can trap solvents/block pores | Maximizes active surface area for reactions |
| Chemical Purity | Oxygen-free environment prevents oxidation | Exposure to hot air causes unwanted oxidation | Preserves the chemical state of precursors |
| Contamination | Sealed system isolates from airborne impurities | Exposed to lab humidity and contaminants | Ensures high reproducibility and stability |
| Material Form | Results in loose, open powder structures | Risk of dense, inactive clumping | Enhances catalytic efficiency and reactivity |
Elevate Your Photocatalyst Synthesis with KINTEK
Don't let structural collapse or oxidation compromise your material's reactivity. Backed by expert R&D and precision manufacturing, KINTEK offers high-performance vacuum ovens designed to meet the rigorous demands of nanostructure preservation. Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, our lab high-temp furnaces are fully customizable for your unique research needs.
Ready to optimize your drying stage? Contact KINTEK today to find your custom solution!
Visual Guide
References
- Lekan Taofeek Popoola, Sabitu Babatunde Olasupo. Photocatalytic degradation of methylene blue dye by magnetized TiO2-silica nanoparticles from rice husk. DOI: 10.1007/s13201-023-02052-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What safety measures are important for vacuum annealing furnaces? Ensure Reliable Operation and Protect Your Lab
- What technical requirements must a furnace meet for Inconel 718 hardening? Master Precision Aging & Cooling
- Why is Titanium sponge used as a chemical getter in high-temperature vacuum distillation? Ensure Ultra-High Metal Purity
- How does the low-pressure environment of an RH vacuum refining furnace influence the morphology of a supersonic jet?
- What are the process advantages of RTT vs. vacuum annealing for nickel-silicon? Achieve precise sub-micron control
- How are active connection parts in a vacuum furnace sealed? Discover the Role of O-Rings and Water Cooling
- What feature of vacuum furnaces makes them suitable for large-scale manufacturing? Unmatched Scalability & Reproducibility
- What are some examples of vacuum brazing projects? Discover High-Strength Joining for Aerospace and Medical



















