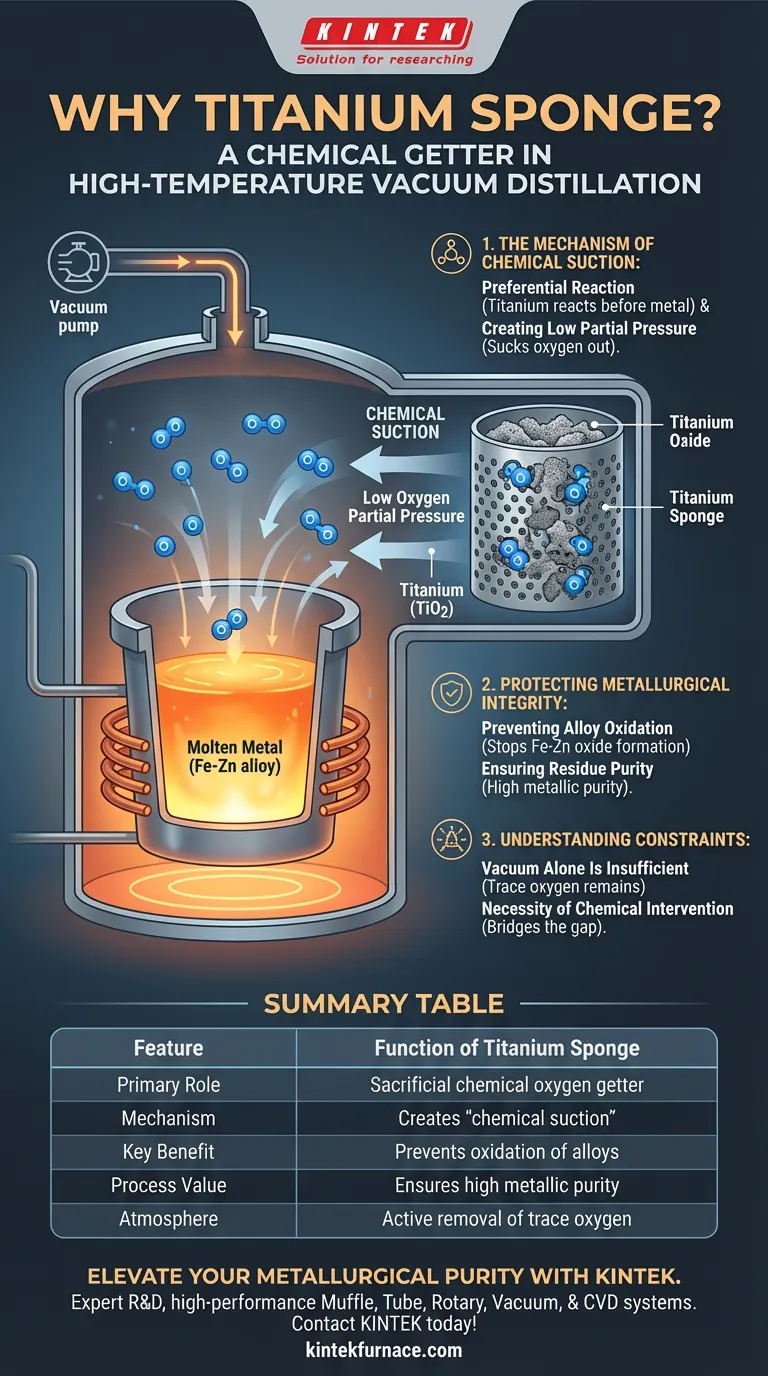
Titanium sponge is utilized primarily for its aggressive affinity for oxygen. In high-temperature vacuum distillation processes, it serves as a sacrificial agent that actively reacts with residual oxygen molecules. This prevents that oxygen from contaminating the metal alloys being processed, ensuring the final output remains pure.
By acting as a chemical oxygen getter, titanium sponge creates "chemical suction" that drastically reduces oxygen partial pressure. This is essential for preventing the oxidation of sensitive metals, ensuring recovered materials maintain high metallic purity.
The Mechanism of Chemical Suction
Preferential Reaction
Titanium sponge does not function passively; it acts as a chemical trap.
Because titanium has a strong thermodynamic preference for oxygen, it reacts with residual gas molecules before they can interact with the target metals.
Creating Low Oxygen Partial Pressure
The primary goal in this context is to lower the oxygen partial pressure beyond what mechanical vacuum pumping might achieve alone.
The reference describes this as "chemical suction." The titanium effectively sucks oxygen out of the system's atmosphere by binding it chemically.
Protecting Metallurgical Integrity
Preventing Alloy Oxidation
High temperatures increase the reactivity of metals, making them susceptible to oxidation even in a vacuum.
Titanium sponge is specifically used to protect alloys, such as Iron-Zinc (Fe-Zn) systems. By intercepting oxygen, it ensures these alloys remain in their metallic state rather than converting to oxides.
Ensuring Residue Purity
The ultimate value of the distillation process relies on the quality of the recovered metals and residues.
The presence of the getter ensures that the final products maintain high levels of metallic purity, free from oxide contamination.
Understanding the Constraints
Why Vacuum Alone Is Insufficient
You might ask why the vacuum pump itself is not enough to protect the metal.
The use of a getter implies that mechanical evacuation leaves behind trace residual oxygen that is still dangerous to the process.
The Necessity of Chemical Intervention
Relying solely on pressure reduction acts as a limitation in high-purity metallurgy.
The "trade-off" here is the requirement for an active chemical participant—the titanium sponge—to bridge the gap between a standard vacuum and the ultra-low oxygen environment required for pure metal recovery.
Making the Right Choice for Your Goal
To maximize the effectiveness of your vacuum distillation process, consider the following specific applications:
- If your primary focus is preventing oxidation: Implement titanium sponge to intercept residual oxygen specifically for sensitive alloys like Fe-Zn.
- If your primary focus is final purity: Use the getter to drive oxygen partial pressure down via chemical suction, ensuring residues meet strict metallic purity standards.
Ultimately, the titanium sponge acts as the critical safeguard that transforms a standard vacuum environment into a high-purity metallurgical zone.
Summary Table:
| Feature | Function of Titanium Sponge |
|---|---|
| Primary Role | Sacrificial chemical oxygen getter |
| Mechanism | Creates "chemical suction" to reduce oxygen partial pressure |
| Key Benefit | Prevents oxidation of sensitive alloys (e.g., Fe-Zn) |
| Process Value | Ensures high metallic purity in recovered residues |
| Atmosphere | Active removal of trace oxygen beyond mechanical vacuum limits |
Elevate Your Metallurgical Purity with KINTEK
Don't let residual oxygen compromise your material integrity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed for precision thermal processing. Whether you need specialized getters or customizable high-temp furnaces for unique distillation needs, our team is ready to assist.
Maximize your lab's efficiency and achieve superior metallic purity — Contact KINTEK today!
Visual Guide
References
- Joongseok Kim, Kyung‐Woo Yi. Investigation of Low-Temperature Molten Oxide Electrolysis of a Mixture of Hematite and Zinc Oxide. DOI: 10.3390/ma18174116
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Ultra High Vacuum Stainless Steel KF ISO CF Flange Pipe Straight Pipe Tee Cross Fitting
People Also Ask
- Why are vacuum and modified atmosphere furnaces essential for 3D printing? Unlock Dense, Strong Parts with Controlled Sintering
- What is the maximum vacuum level for a high vacuum furnace? Achieve Ultra-Clean Processing for Advanced Materials
- What industries commonly use vacuum furnaces? Essential for Aerospace, Medical, Automotive, and Electronics
- What core technical conditions does a high-temperature vacuum resistance furnace provide for molten steel infiltration?
- What is the core role of a laboratory vacuum furnace in the carbothermic reduction process for magnesium? Creating the Ideal Environment for High-Purity Production
- Why is a high-precision vacuum sealing system necessary for CrSb? Ensure Pure Crystal Growth & Prevent Oxidation
- What role do vacuum annealing furnaces play in optical material processing? Enhance Clarity and Performance for Your Optics
- What core role does a high-temperature vacuum sintering furnace play in Sm:YAG ceramics? Mastering Optical Clarity



















