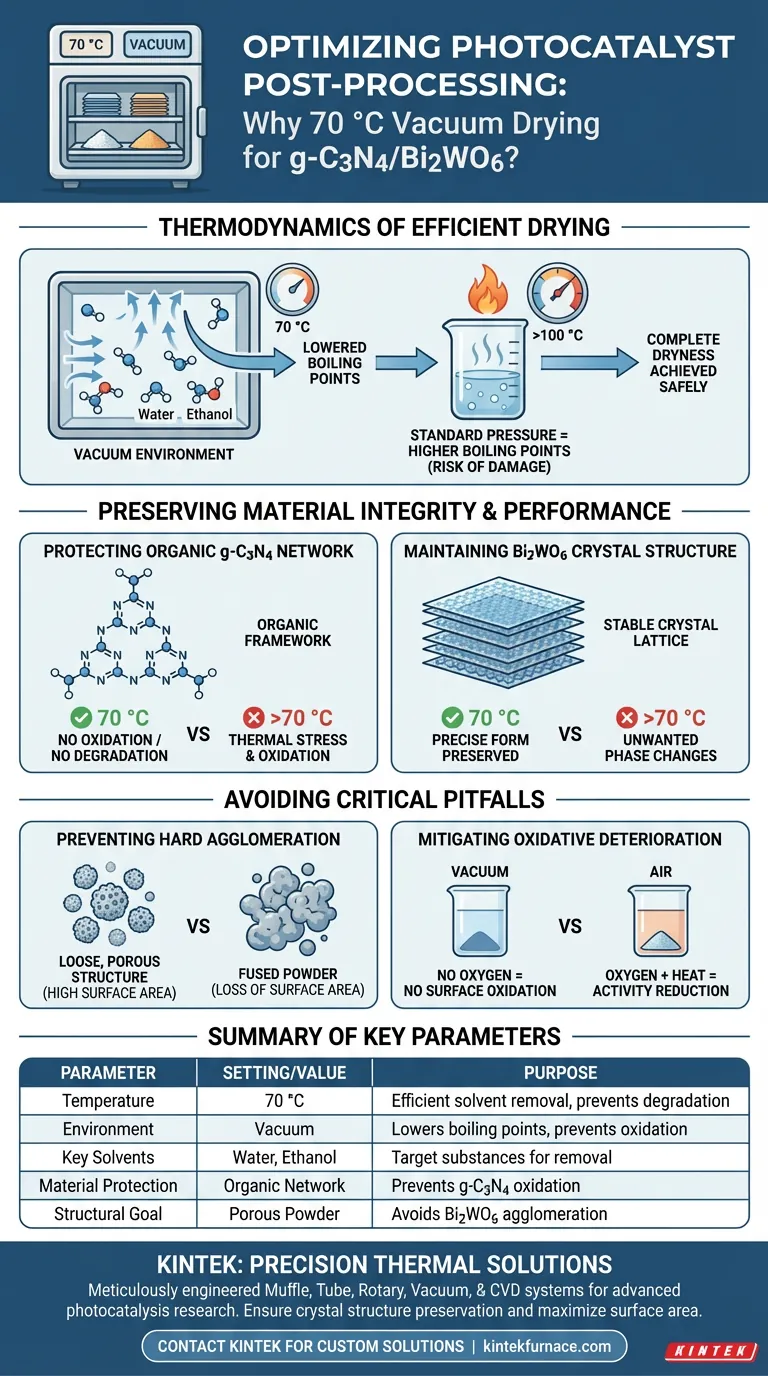
Setting the vacuum drying oven to 70 °C allows for the efficient removal of residual moisture and anhydrous ethanol while strictly preserving the structural integrity of the g-C3N4/Bi2WO6 photocatalyst. This specific temperature acts as a safe threshold that facilitates evaporation under reduced pressure but remains low enough to prevent thermal degradation or oxidation of the composite material.
Core Takeaway By combining a moderate temperature of 70 °C with a vacuum environment, you lower the boiling point of solvents to achieve complete dryness without subjecting the material to destructive heat. This safeguards the organic network of g-C3N4 and preserves the high surface area of the Bi2WO6 nanosheets, preventing the loss of photocatalytic activity that occurs with high-temperature agglomeration.
The Role of Thermodynamics in Post-Processing
Lowering Solvent Boiling Points
The primary mechanism at work is the relationship between pressure and boiling points. By utilizing a vacuum environment, the boiling points of residual solvents—specifically water and anhydrous ethanol—are significantly reduced.
This allows these solvents to evaporate rapidly at 70 °C. Under normal atmospheric pressure, removing these solvents would require much higher temperatures, which could be detrimental to the sample.
Ensuring Complete Dryness
The combination of vacuum and steady heat ensures the catalyst reaches a state of complete dryness.
Removing every trace of solvent is critical for accurate weight measurement and performance testing. The vacuum ensures that solvent molecules trapped deep within the pores of the material are extracted effectively.
Preserving Material Integrity
Protecting the g-C3N4 Organic Network
Graphitic carbon nitride (g-C3N4) possesses an organic network that can be sensitive to thermal stress.
Drying at 70 °C prevents the oxidation of this organic framework. Higher temperatures, particularly in the presence of air, could degrade the network, altering its band gap and reducing its photocatalytic efficiency.
Maintaining Bi2WO6 Crystal Structure
Bismuth tungstate (Bi2WO6) often takes the form of 2D nanosheets. The 70 °C set point ensures the crystal structure of these nanosheets remains stable and does not undergo unwanted phase changes.
Preserving the precise crystallographic form is essential, as the material’s electronic properties rely heavily on its specific crystal lattice arrangement.
Understanding the Trade-offs
Avoiding Hard Agglomeration
A critical pitfall in drying nanomaterials is "hard agglomeration." This occurs when high temperatures cause powder particles to fuse together irreversibly.
By limiting the temperature to 70 °C, the process maintains a loose, porous structure. This preserves the fine micro-nano structure and ensures the high surface area required for effective catalytic reactions is not lost to clumping.
Preventing Oxidative Deterioration
High-activity nanocatalysts are prone to oxidative deterioration if exposed to heat and oxygen simultaneously for prolonged periods.
The vacuum oven mitigates this risk by removing oxygen from the chamber. If you were to dry these materials at 70 °C in a standard air oven, you would likely see a reduction in activity due to surface oxidation.
Making the Right Choice for Your Goal
When finalizing your post-processing protocol, consider the specific requirements of your analysis:
- If your primary focus is Structural Purity: Adhere strictly to the 70 °C limit to prevent thermal defects in the organic g-C3N4 network.
- If your primary focus is Maximizing Surface Area: Ensure the vacuum pressure is stable to prevent pore collapse and avoid hard agglomeration of the Bi2WO6 nanosheets.
Ultimately, the 70 °C vacuum drying protocol is the optimal compromise that yields a dry, pure powder without sacrificing the delicate 2D architecture that drives photocatalytic performance.
Summary Table:
| Parameter | Setting/Value | Purpose in Post-Processing |
|---|---|---|
| Temperature | 70 °C | Efficient solvent removal without thermal degradation |
| Environment | Vacuum | Lowers solvent boiling points & prevents oxidation |
| Key Solvents | Water, Ethanol | Target substances for removal during drying |
| Material Protection | Organic Network | Prevents oxidation of g-C3N4 framework |
| Structural Goal | Porous Powder | Avoids hard agglomeration of Bi2WO6 nanosheets |
Precision Thermal Processing for Advanced Photocatalysis
Maintaining the delicate 2D architecture of materials like g-C3N4/Bi2WO6 requires specialized equipment that offers uncompromising temperature uniformity and atmosphere control.
KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems, all meticulously engineered for researchers and manufacturers who demand excellence. Backed by expert R&D and manufacturing, our high-temp lab furnaces are fully customizable to meet your unique materials science needs—ensuring you preserve crystal structures and maximize surface area every time.
Ready to elevate your lab's performance?
Contact KINTEK Today to Find Your Custom Solution
Visual Guide
References
- Wenxing Chen, Huilin Hou. Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction. DOI: 10.3390/coatings15010032
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- What critical function does argon gas serve in sintering Ti2AlN ceramics? Ensure Phase Purity and Prevent Oxidation
- How does the sealed shell of a vacuum furnace contribute to its functionality? Unlock High-Purity Thermal Processing
- What is the purpose of the water cooling system in a vacuum furnace? Ensure Safety and Efficiency in High-Temp Operations
- What are the steps involved in a typical vacuum brazing treatment? Master the Process for Strong, Clean Joints
- What are the benefits of vacuum brazing over welding? Preserve Material Integrity and Achieve Clean Joints
- What are the advantages of using a vacuum furnace for heat treatment? Achieve Superior Process Control and Clean Results
- What are the selection criteria for vacuum pumps in vacuum furnaces? Optimize for Purity and Efficiency
- What is the working principle of a vacuum furnace? Achieve High-Purity Heat Treatment for Sensitive Materials



















