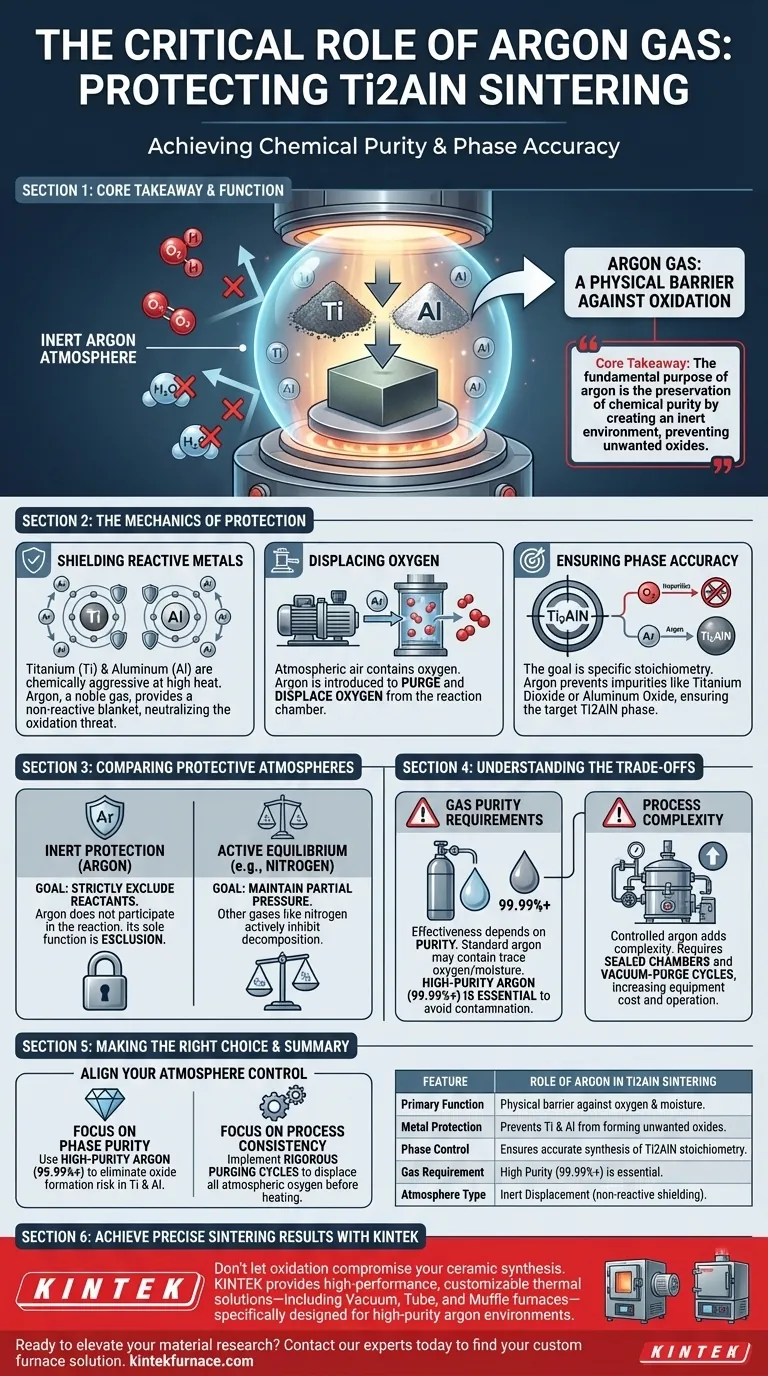
Argon gas serves as a critical physical barrier against oxidation during the sintering of Ti2AlN ceramics. It acts as an inert medium that displaces air within the reaction chamber, preventing the highly reactive titanium (Ti) and aluminum (Al) powders from chemically bonding with oxygen.
Core Takeaway The fundamental purpose of argon in this process is the preservation of chemical purity. By creating an inert environment, argon prevents the constituent metals from degrading into unwanted oxides, ensuring the successful and accurate synthesis of the target Ti2AlN phase.

The Mechanics of Protection
Shielding Reactive Metals
Titanium (Ti) and aluminum (Al) are the primary metallic components in the synthesis of Ti2AlN. Both of these metals are chemically aggressive, particularly when heated.
In the presence of oxygen, these metals rapidly oxidize. Argon, being a noble gas, provides a non-reactive blanket that surrounds these powders, neutralizing the threat of oxidation.
Displacing Oxygen
The reaction chamber naturally contains atmospheric air, which is rich in oxygen. Before and during the high-temperature sintering process, argon is introduced to fill the chamber.
This effectively purges oxygen from the environment. Without this displacement, the oxygen would immediately react with the heated metal powders.
Ensuring Phase Accuracy
The ultimate goal of sintering is to achieve a specific stoichiometry: the Ti2AlN phase. If oxidation occurs, the chemical balance is disrupted.
Instead of Ti2AlN, the reaction would yield impurities like titanium dioxide or aluminum oxide. Argon ensures the reaction yields only the intended ceramic compound.
Comparing Protective Atmospheres
Inert vs. Active Protection
It is important to distinguish between inert protection and active equilibrium maintenance. As seen in other ceramic processes, such as silicon nitride sintering, gases like nitrogen are used to actively inhibit decomposition by maintaining partial pressure.
The Specific Role of Argon
However, for Ti2AlN, the goal is not to balance a decomposition pressure, but to strictly exclude reactants. Argon does not participate in the chemical reaction in any way; its sole function is exclusion.
Understanding the Trade-offs
Gas Purity Requirements
While argon is chemically inert, the effectiveness of the protective atmosphere depends entirely on the purity of the gas source. Standard industrial argon may still contain trace amounts of oxygen or moisture.
If the argon supply is not of sufficiently high purity, even the "protective" atmosphere can introduce enough oxygen to contaminate the sensitive titanium and aluminum powders.
Process Complexity
Using a controlled argon atmosphere adds complexity to the furnace setup. The chamber must be sealed capable of maintaining a positive pressure or vacuum-purge cycles.
This increases the equipment cost and operational overhead compared to sintering processes that can be performed in air.
Making the Right Choice for Your Goal
To optimize the sintering of Ti2AlN, you must align your atmosphere control with your specific purity requirements.
- If your primary focus is Phase Purity: Use high-purity argon (99.99%+) to completely eliminate the risk of oxide formation in Ti and Al powders.
- If your primary focus is Process Consistency: Implement rigorous purging cycles to ensure all atmospheric oxygen is displaced by argon before heat is applied.
Success in sintering Ti2AlN relies not just on temperature control, but on the absolute exclusion of oxygen through a high-quality inert argon atmosphere.
Summary Table:
| Feature | Role of Argon in Ti2AlN Sintering |
|---|---|
| Primary Function | Acts as a physical barrier against oxygen and moisture. |
| Metal Protection | Prevents reactive Ti and Al powders from forming unwanted oxides. |
| Phase Control | Ensures the accurate synthesis of the target Ti2AlN stoichiometry. |
| Gas Requirement | High purity (99.99%+) is essential to avoid trace contamination. |
| Atmosphere Type | Inert displacement (non-reactive shielding). |
Achieve Precise Sintering Results with KINTEK
Don't let oxidation compromise your ceramic synthesis. KINTEK provides high-performance, customizable thermal solutions—including Vacuum, Tube, and Muffle furnaces—specifically designed to maintain the high-purity argon environments required for sensitive materials like Ti2AlN.
Backed by expert R&D and world-class manufacturing, our systems ensure your lab achieves perfect phase accuracy and process consistency every time.
Ready to elevate your material research? Contact our experts today to find your custom furnace solution.
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- What is the primary function of a vacuum furnace? Achieve Purity and Precision in Heat Treatment
- How does a vacuum annealing furnace contribute to microstructural recovery of ODS steel? Unlock Material Performance
- What is the significance of the vacuum drying process for ultrafine cemented carbide? Preserve Powder Purity & Quality
- What are the benefits of the degassing effect during vacuum heating? Unlock Superior Metal Performance and Durability
- Why must humidity be controlled in aluminum alloy furnaces? Prevent Blistering & Hydrogen Damage Now
- How does dew point monitoring influence the process control of sintering furnaces in MIM? Ensure Peak Material Quality
- Why is a vacuum drying oven set to 70 °C for g-C3N4/Bi2WO6? Optimize Your Photocatalyst Post-Processing
- Why is a high-temperature homogenization furnace treatment necessary for (CoCrNi)94Al3Ti3 alloys? Ensure Metal Purity



















