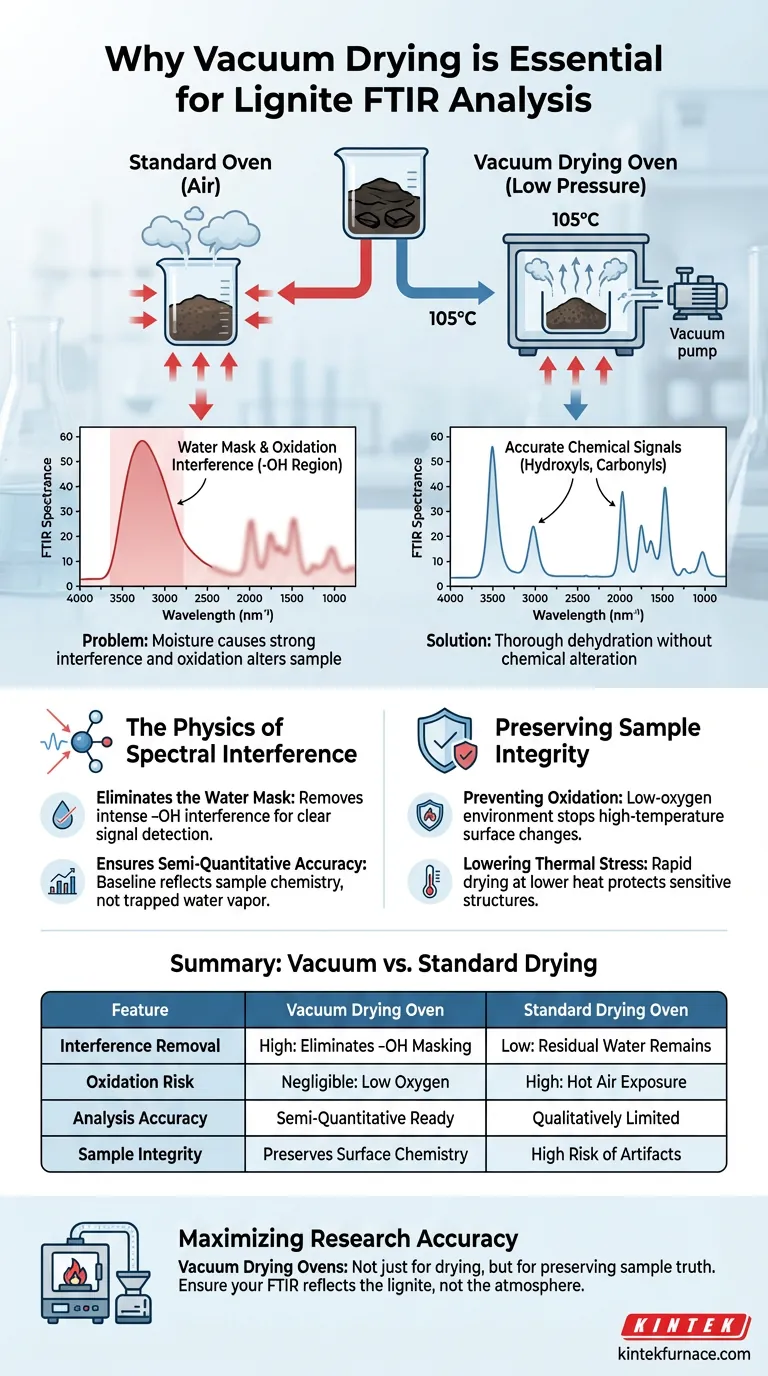
The use of a vacuum drying oven is essential to eliminate physically adsorbed water that creates significant spectral noise during analysis. For lignite samples undergoing Fourier Transform Infrared (FTIR) testing, this process is critical because residual moisture causes strong absorption interference in the hydroxyl (-OH) region, effectively masking the chemical signals you need to observe.
Core Takeaway Vacuum drying solves the "water masking" problem by removing moisture without chemically altering the sample. It creates a low-pressure environment that allows for thorough dehydration while preventing the high-temperature oxidation that would occur in a standard oven.

The Physics of Spectral Interference
Eliminating the Water Mask
Lignite is a porous material that naturally retains physically adsorbed water. In FTIR spectroscopy, water molecules absorb infrared radiation intensely, particularly in the hydroxyl (-OH) region.
If this water is not removed, its broad absorption peaks will overlap with the signals from the lignite's own surface functional groups. This makes it impossible to distinguish between the moisture content and the sample's actual hydrogen bonds or free hydroxyls.
Ensuring Semi-Quantitative Accuracy
To perform a valid semi-quantitative analysis, the baseline of the spectrum must be attributable solely to the sample material.
By dehydrating the lignite (typically at 105°C), the vacuum oven ensures that the resulting spectrum accurately reflects the evolution of the lignite's chemical structure, rather than the behavior of trapped water vapor.
Preserving Sample Integrity
Preventing Oxidation
Standard drying ovens rely on hot air, which poses a risk to organic materials like lignite. Heating lignite in the presence of oxygen can induce oxidation, artificially altering the surface functional groups before the test even begins.
A vacuum oven operates in a low-oxygen environment. This prevents high-temperature oxidation, ensuring that the detected peaks (such as carbonyls or hydroxyls) reflect the true chemical state of the material, not artifacts of the drying process.
Lowering Thermal Stress
The vacuum environment significantly lowers the boiling point of water and other volatiles.
This allows moisture to be removed from the deep pores of the lignite more rapidly and at potentially lower thermal energy levels. This is crucial for preventing thermal degradation or framework collapse in structurally sensitive components of the sample.
Understanding the Trade-offs
Processing Time vs. Data Quality
Using a vacuum oven adds a layer of complexity compared to simple air drying. It requires managing vacuum pumps and seal integrity, which can slightly increase the setup time for sample preparation.
Sensitivity to Volatiles
While excellent for removing water, the vacuum process is indiscriminate regarding volatiles. If your specific lignite study aims to analyze certain volatile organic compounds (VOCs) that have low boiling points, aggressive vacuum drying might strip these away alongside the water.
Making the Right Choice for Your Goal
When preparing lignite for FTIR, the drying method dictates the reliability of your data.
- If your primary focus is Semi-Quantitative Analysis: You must use a vacuum oven to completely remove water interference in the -OH region for accurate integration calculations.
- If your primary focus is Surface Chemistry Characterization: You must use a vacuum oven to prevent oxidation artifacts from appearing in your spectrum.
- If your primary focus is Speed: A standard oven is faster but will likely yield unusable data for detailed hydroxyl analysis due to oxidation and residual moisture.
The vacuum drying oven is not just a drying tool; it is a sample preservation device that ensures the FTIR spectrum represents the lignite, not the atmosphere.
Summary Table:
| Feature | Vacuum Drying Oven | Standard Drying Oven |
|---|---|---|
| Interference Removal | High: Eliminates -OH masking | Low: Residual water remains |
| Oxidation Risk | Negligible (Low Oxygen) | High (Hot Air Exposure) |
| Analysis Accuracy | Semi-Quantitative Ready | Qualitatively Limited |
| Sample Integrity | Preserves surface chemistry | High risk of thermal artifacts |
Maximize Your Research Accuracy with KINTEK
Don't let moisture and oxidation compromise your FTIR results. At KINTEK, we understand that precise sample preparation is the foundation of scientific discovery. Backed by expert R&D and world-class manufacturing, we provide high-performance Vacuum, Muffle, Tube, and CVD systems tailored for lab-scale and industrial needs.
Whether you require customizable high-temperature furnaces or specialized vacuum drying solutions for lignite and other porous materials, our team is ready to deliver the reliability you deserve.
Ready to elevate your lab's efficiency? Contact us today to find your custom solution!
Visual Guide
References
- Baoshan Jia, Xian Wu. Effects of pre-oxidation temperature and air volume on oxidation thermogravimetric and functional group change of lignite. DOI: 10.1371/journal.pone.0316705
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- Why is vacuum carburizing suitable for high-performance carburizing steels? Achieve Superior Hardening with Precision Control
- What are the key features of vacuum performance customization? Achieve Precise Control for Your Lab Processes
- What maintenance is required for vacuum furnace heating elements? Ensure Reliability and Prevent Costly Downtime
- What services are offered for vacuum brazing? Partner for Precision Joining Solutions
- What is the purpose of vacuum sintering furnaces? Achieve High-Purity, Dense Materials
- What are the two types of vacuum furnaces based on heating form? Choose the Right Design for Your Lab
- What is the difference between an atmosphere furnace and a vacuum furnace? Choose the Right Heat Treatment for Your Lab
- What are some applications of vacuum brazing? Achieve Strong, Clean Joints in Aerospace and More



















