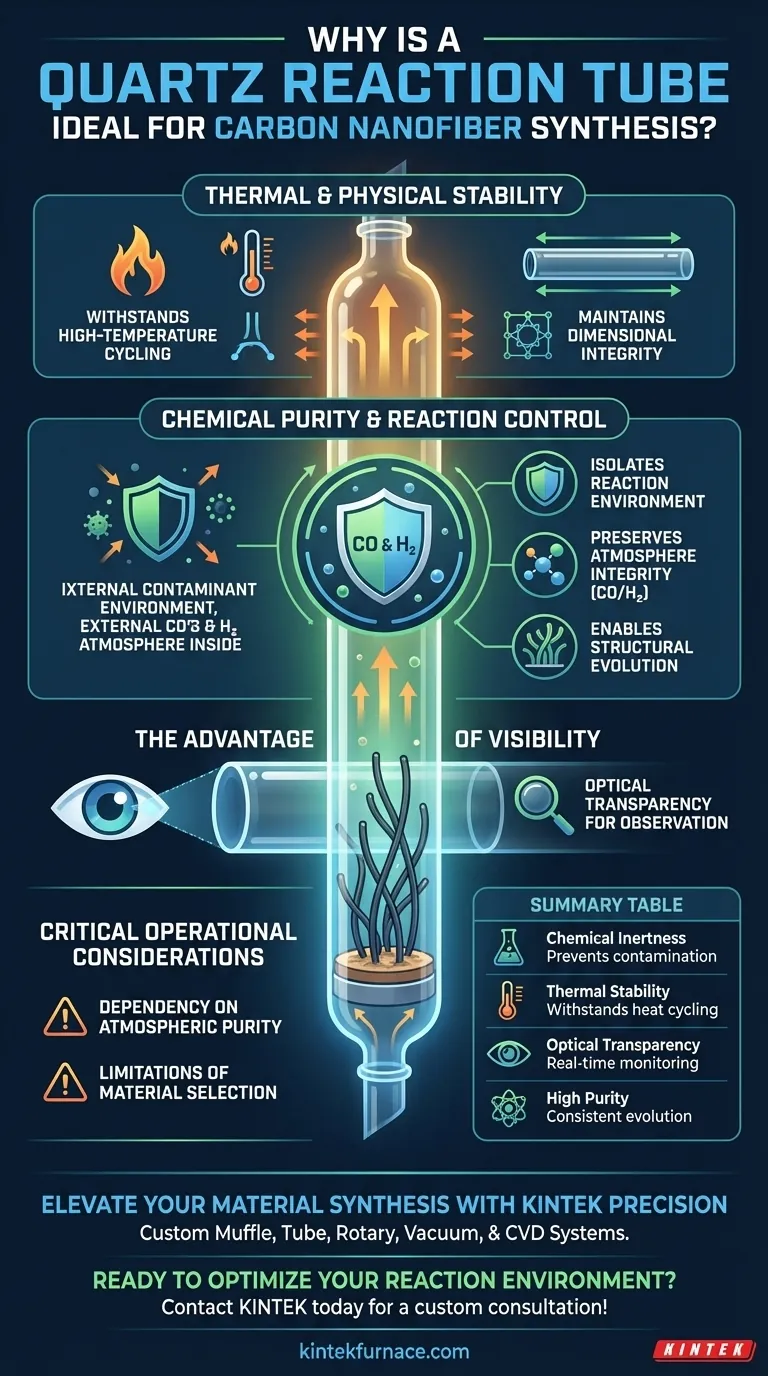
A quartz reaction tube serves as the definitive reaction chamber for preparing tubular carbon nanofibers due to its unique ability to maintain chemical inertness under extreme heat. It provides a stable, contaminant-free environment that is essential for the decomposition of carbon monoxide (CO) and the subsequent structural formation of the nanofibers.
Core Takeaway Quartz acts as a high-purity isolation barrier, ensuring that the sensitive carbon monoxide and hydrogen atmosphere remains uncontaminated during synthesis. Its combination of thermal resilience and optical transparency allows for precise control and monitoring of the nanofiber's directional deposition.

Thermal and Physical Stability
Withstanding High-Temperature Cycling
The synthesis of carbon nanofibers requires subjecting the reaction chamber to intense heat fluctuations. A quartz tube is specifically selected because it can endure this high-temperature cycling without structural failure.
Maintaining Dimensional Integrity
During the reaction, the chamber must remain stable to ensure consistent processing conditions. Quartz maintains excellent thermal stability, preventing warping or degradation that could alter the reaction dynamics inside the tube.
Chemical Purity and Reaction Control
Isolating the Reaction Environment
The most critical function of the quartz tube is providing high chemical purity. It effectively acts as a barrier, isolating the internal reaction from the external environment.
Preserving Atmosphere Integrity
For tubular carbon nanofibers to form correctly, the reaction requires a specific mixture of pure CO and H2 (hydrogen). The quartz material prevents contaminants from leaching into the chamber, ensuring the atmosphere remains conducive to CO decomposition.
Enabling Structural Evolution
The purity of the environment directly impacts the quality of the final product. By maintaining a pristine atmosphere, the quartz tube ensures that the carbon nanofibers can complete their directional deposition and structural evolution without interference.
The Advantage of Visibility
Optical Transparency for Observation
Beyond its thermal and chemical properties, quartz offers the distinct advantage of optical transparency. This allows researchers to visually monitor the reaction progress and the status of the substrate in real-time.
Critical Operational Considerations
Dependency on Atmospheric Purity
While quartz is the ideal material for the vessel, it is not a passive component; its primary value lies in isolation. The "ideal" nature of the chamber is entirely dependent on the integrity of the CO/H2 mixture introduced; the tube preserves purity but cannot generate it.
Limitations of Material Selection
The text highlights that the structural evolution of the nanofibers is linked to this specific environment. Consequently, substituting quartz with materials that possess lower chemical purity or thermal stability would likely introduce impurities, disrupting the directional deposition process.
Making the Right Choice for Your Goal
To maximize the quality of your carbon nanofiber synthesis, align your equipment choice with your specific technical requirements.
- If your primary focus is process monitoring: Prioritize quartz for its optical transparency, which permits direct observation of the reaction stages without breaking the seal.
- If your primary focus is structural integrity of the fiber: Rely on the high chemical purity of quartz to maintain the strict CO/H2 atmosphere required for precise directional deposition.
Success in nanofiber preparation relies on the uncompromised isolation of the reaction environment.
Summary Table:
| Feature | Benefit for Carbon Nanofiber Synthesis |
|---|---|
| Chemical Inertness | Prevents contamination and preserves CO/H2 atmosphere integrity |
| Thermal Stability | Withstands high-temperature cycling without warping or failure |
| Optical Transparency | Enables real-time visual monitoring of directional deposition |
| High Purity | Ensures consistent structural evolution and material quality |
Elevate Your Material Synthesis with KINTEK Precision
Achieve uncompromising results in your carbon nanofiber research with KINTEK’s high-performance quartz solutions. Backed by expert R&D and world-class manufacturing, KINTEK offers customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of lab high-temperature processing. Whether you require superior thermal resilience or pristine chemical isolation, our equipment is engineered to your unique specifications.
Ready to optimize your reaction environment? Contact KINTEK today for a custom consultation!
Visual Guide
References
- Minki Sung, Seong‐Ho Yoon. Preparation Uniform Thin Tubular Carbon Nanofiber Using Novel Bimetallic Catalyst at Low Temperature and Its Structural Feature. DOI: 10.1021/acsomega.4c10295
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What key functions do graphite molds perform in RuTi alloy sintering? Achieve High-Density Precision at 1000°C
- What function does a laboratory blast drying oven perform? Optimize Pretreatment for Magnetic Particles
- Why are graphite crucible furnaces used in vacuum or protective atmosphere environments? Prevent Oxidation and Ensure Purity
- Why is a heating device with magnetic stirring required for Y2O3-MgO precursors? Ensure Perfect Particle Coating
- Why is a high-pressure MFC necessary for CHP systems? Achieve Precision in Catalytic Hydropyrolysis Data
- Why is precise temperature sensor placement critical in high-temp viscometers? Expert Insights for Accurate Melt Data
- Why is an alumina crucible required for bauxite residue thermal analysis? Ensure Stability and Data Purity Up to 1400°C
- Why use high-precision gas flow control for argon in oil migration simulations? Achieve Re=215 Accuracy



















