Effective processing of synthesized nanomaterials relies heavily on controlled post-synthesis treatment. A laboratory drying oven is strictly necessary to eliminate residual moisture adhering to the surface of cobalt-zirconium co-doped iron oxide nanoparticles following the washing process. This controlled environment is critical for preventing the degradation of the material's physical and chemical properties before it can be utilized or analyzed.
The core purpose of the drying oven is not simply dehydration; it is structural preservation. By removing moisture under regulated conditions, you prevent irreversible agglomeration and surface oxidation, ensuring the nanopowder retains the fluidity and purity required for accurate characterization.

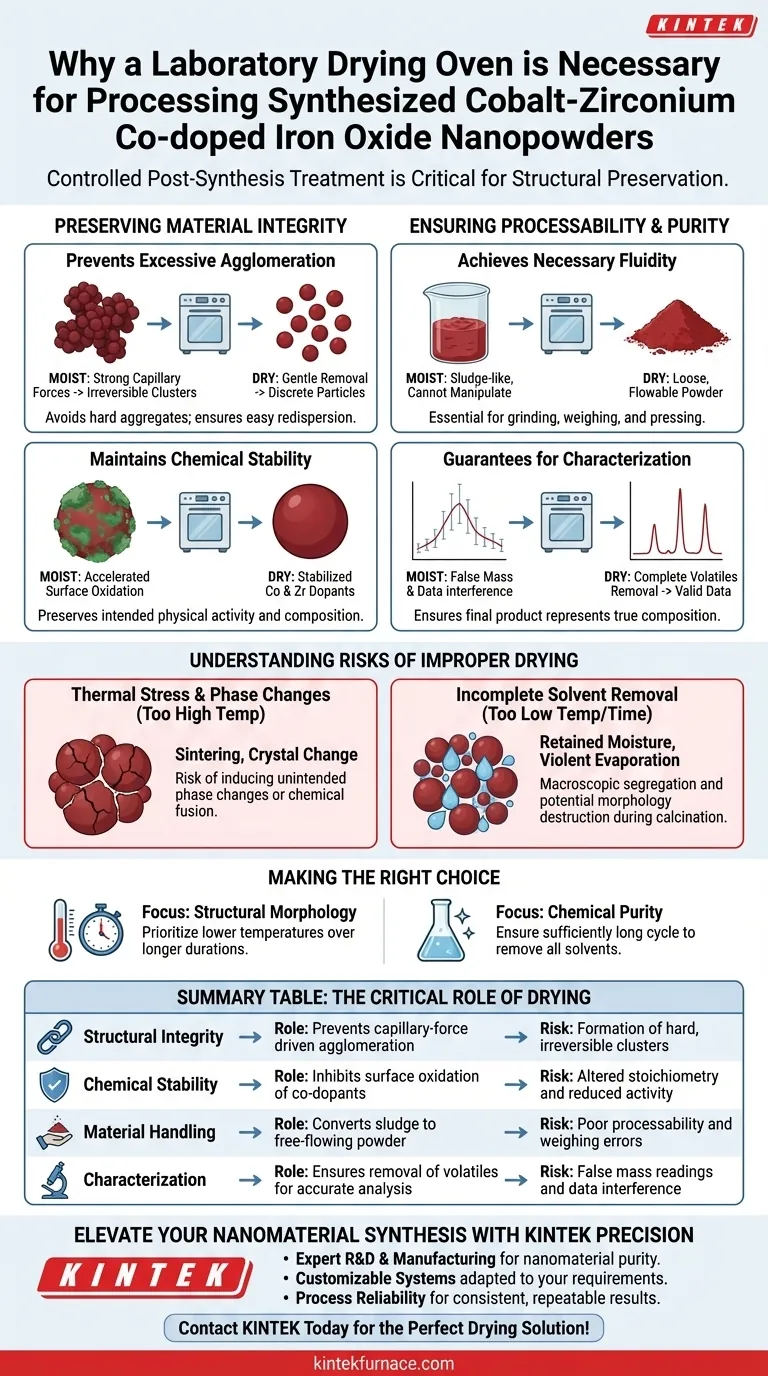
Preserving Material Integrity
preventing Excessive Agglomeration
Nanoparticles have high surface energy and are naturally prone to clumping. Residual moisture acts as a binding agent, creating capillary forces that pull particles together into large, irregular clusters.
A laboratory drying oven removes this moisture gently. This ensures that the particles remain discrete rather than fusing into hard aggregates that are difficult to redisperse.
Maintaining Chemical Stability
Iron oxide-based materials can be sensitive to environmental conditions. Leaving the nanopowders moist for extended periods can accelerate surface oxidation or unwanted chemical reactions.
Rapid, controlled drying mitigates this risk. It stabilizes the chemical composition of the cobalt and zirconium co-dopants, preserving the material's intended physical activity.
Ensuring Processability and Purity
Achieving Necessary Fluidity
For a nanopowder to be useful, it must possess specific handling characteristics. A moist powder is sludge-like and impossible to manipulate precisely.
The drying process transforms the washed precipitate into a loose, flowable powder. This fluidity is essential for subsequent processing steps, such as grinding, weighing, or pressing.
Guarantees for Characterization
Analytical techniques require high purity to yield valid data. Any remaining solvent or water contributes false mass and can interfere with spectroscopic or thermal analysis.
By ensuring the complete removal of volatiles, the drying oven guarantees that the final product represents the true composition of the synthesized material.
Understanding the Risks of Improper Drying
While the drying oven is essential, the parameters must be chosen carefully to avoid unintended side effects.
Thermal Stress and Phase Changes
If the drying temperature is set too high, you risk inducing phase changes in the iron oxide crystal structure. Excessive heat can also cause "sintering," where particles fuse chemically rather than just physically clumping.
Incomplete Solvent Removal
Conversely, insufficient drying time or temperature results in retained moisture. This can lead to macroscopic segregation of components or violent evaporation during later high-temperature calcination, potentially destroying the material's morphology.
Making the Right Choice for Your Goal
To maximize the quality of your cobalt-zirconium co-doped iron oxide nanopowders, tailor your drying approach to your specific analytical needs.
- If your primary focus is structural morphology: Prioritize lower temperatures over longer durations to remove moisture without inducing thermal sintering or particle growth.
- If your primary focus is chemical purity: Ensure the drying cycle is sufficiently long to remove all traces of washing solvents, which ensures accurate stoichiometric analysis.
Controlled drying is the critical bridge between raw chemical synthesis and a reliable, functional nanomaterial.
Summary Table:
| Process Objective | Role of Drying Oven | Risk of Omission |
|---|---|---|
| Structural Integrity | Prevents capillary-force driven agglomeration | Formation of hard, irreversible clusters |
| Chemical Stability | Inhibits surface oxidation of co-dopants | Altered stoichiometry and reduced activity |
| Material Handling | Converts sludge to free-flowing powder | Poor processability and weighing errors |
| Characterization | Ensures removal of volatiles for accurate analysis | False mass readings and data interference |
Elevate Your Nanomaterial Synthesis with KINTEK Precision
Maintaining the structural integrity of advanced nanopowders like cobalt-zirconium iron oxide requires the precise thermal control that only expert-engineered equipment can provide. KINTEK empowers researchers and manufacturers with high-performance laboratory solutions, including specialized Muffle, Tube, and Vacuum systems tailored for delicate drying and calcination processes.
Our Value to You:
- Expert R&D & Manufacturing: Access cutting-edge thermal technology designed for nanomaterial purity.
- Customizable Systems: We adapt our furnaces to meet your unique temperature and atmospheric requirements.
- Process Reliability: Ensure consistent, repeatable results to prevent sintering and phase changes.
Don't let improper drying compromise your research. Contact KINTEK today to find the perfect drying solution for your laboratory needs!
Visual Guide
References
- Saba Yaqoob, Alberto D’Amore. Magnetic and Dielectric Properties of Cobalt and Zirconium Co-Doped Iron Oxide Nanoparticles via the Hydrothermal Synthesis Approach. DOI: 10.3390/jcs9010032
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How does a precision drying oven influence ZnO gel drying? Achieve Perfect Microporous Structures
- What is the significance of the thermal environment in calcination? Achieve Pure Ceramic Phases with KINTEK
- How do stirring equipment and temperature-controlled heating stages influence magnetic nanoparticle quality?
- What role does an automatic high-temperature cyclic furnace play in evaluating TBC systems? Validate Durability Now.
- How do water quenching and furnace cooling methods differ in their application to high-entropy alloys? Expert Insights
- What are batch catalytic debinding ovens used for? Speed Up MIM/CIM with Low-Temp Debinding
- Why is vacuum distillation preferred for biodiesel ethanol removal? Protect Fuel Quality with Low-Temp Processing
- What role does a high-temperature thermal simulation system play in the dissolution of precipitates in steel?



















