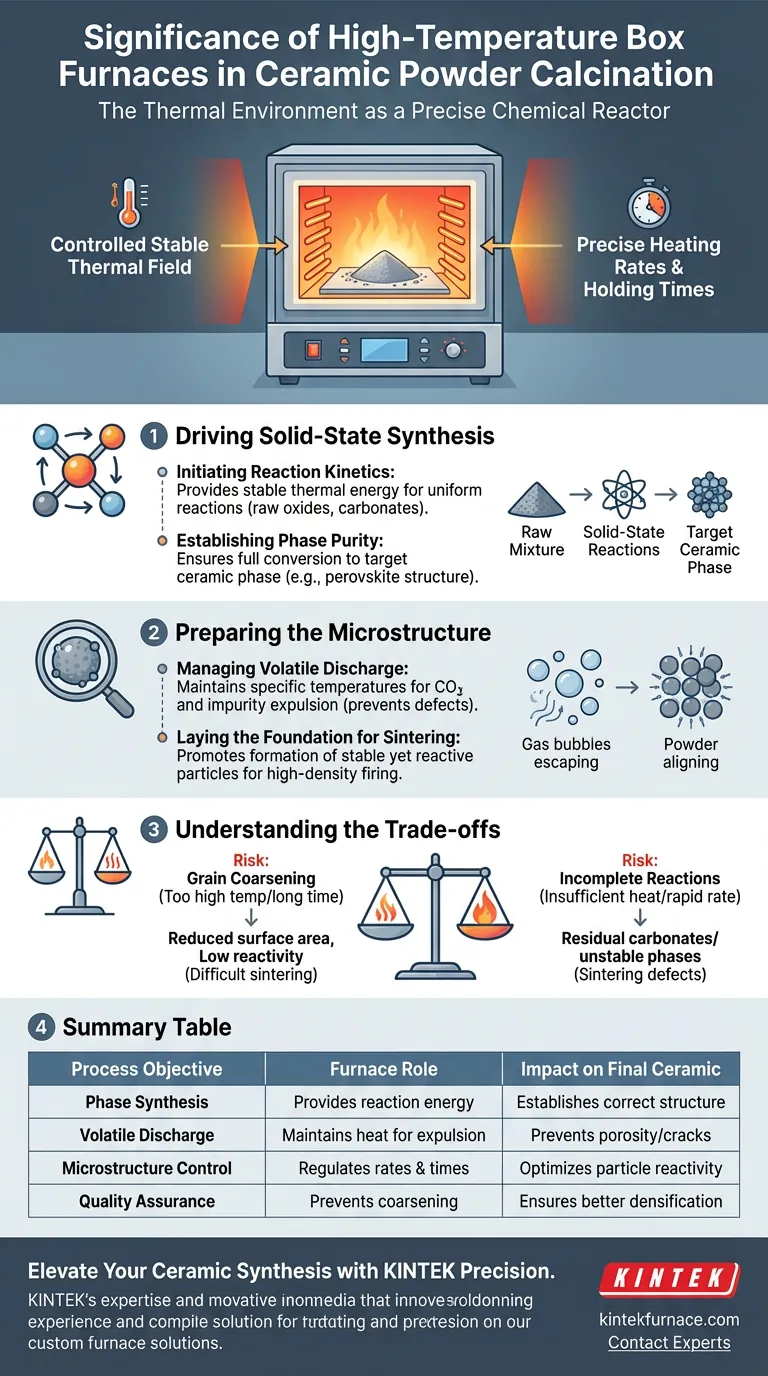
The thermal environment acts as a precise chemical reactor. A high-temperature box furnace generates the stable thermal field necessary to initiate and sustain solid-state reactions between raw oxides and carbonates. This controlled environment ensures the complete discharge of volatile byproducts and the successful formation of the target ceramic phase.
By precisely controlling heating rates and holding times, the furnace converts raw precursors into stable crystalline structures. This calcination step is the critical foundation that determines the success of subsequent densification and sintering.
Driving Solid-State Synthesis
The primary function of the box furnace during calcination is to transition materials from a raw mixture to a reacted compound.
Initiating Reaction Kinetics
The furnace provides a stable thermal field that promotes the initial solid-state reactions.
Raw materials, typically a mix of various oxides and carbonates, require specific energy thresholds to interact. The furnace supplies this energy uniformly to ensure the reaction occurs throughout the entire batch, not just on the surface.
Establishing Phase Purity
The goal of this stage is to form the target ceramic phase, such as a perovskite structure.
Through precise thermal management, the furnace ensures the material fully converts from intermediate phases into a stable, desired crystal structure. This sets the chemical identity of the ceramic before it is ever shaped or densified.
Preparing the Microstructure
Beyond chemistry, the thermal environment dictates the physical quality of the powder.
Managing Volatile Discharge
A critical role of the thermal field is the removal of impurities.
By maintaining specific temperatures, the furnace ensures that volatile components, particularly carbon dioxide evolved from carbonates, are fully discharged. Failure to remove these gases leads to defects, such as porosity or cracking, in the final product.
Laying the Foundation for Sintering
The calcination process prepares the powder for the final densification stage.
The furnace promotes the formation of particles that are chemically stable but still reactive enough to sinter well. This step lays the groundwork for achieving high density in the subsequent firing processes.
Understanding the Trade-offs
While the box furnace is essential for phase formation, improper thermal control can degrade powder quality.
The Risk of Grain Coarsening
If the temperature is too high or the holding time too long, particles may grow too large.
This "premature grain coarsening" reduces the surface area and reactivity of the powder. Low-reactivity powders are difficult to sinter to full density later in the process.
The Danger of Incomplete Reactions
Conversely, insufficient heat or rapid heating rates may leave reactions unfinished.
This results in residual carbonates or unstable phases remaining in the powder. These residuals can release gas during final sintering, causing catastrophic structural failure in the ceramic part.
Making the Right Choice for Your Goal
To maximize the effectiveness of your calcination process, align your furnace settings with your specific material requirements.
- If your primary focus is phase purity: Prioritize a holding time sufficient to ensure the complete exhaustion of carbon dioxide and other volatiles.
- If your primary focus is densification: Optimize the heating rate to form stable particles without inducing premature grain growth that hinders sintering.
The quality of your final ceramic component is chemically predetermined by the stability of the thermal field during calcination.
Summary Table:
| Process Objective | Furnace Role | Impact on Final Ceramic |
|---|---|---|
| Phase Synthesis | Provides energy for solid-state reactions | Establishes correct crystalline structure (e.g., perovskite) |
| Volatile Discharge | Maintains heat to expel CO2 and impurities | Prevents porosity, cracking, and structural defects |
| Microstructure Control | Regulates heating rates and holding times | Optimizes particle reactivity for high-density sintering |
| Quality Assurance | Prevents premature grain coarsening | Ensures maximum surface area for better densification |
Elevate Your Ceramic Synthesis with KINTEK Precision
Don't let inconsistent thermal fields compromise your material integrity. At KINTEK, we understand that the calcination stage is the foundation of ceramic excellence. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to deliver the precise thermal control your research demands.
Whether you are refining phase purity or optimizing grain size, our lab high-temperature furnaces are fully customizable to meet your unique processing needs. Experience the KINTEK advantage in precision and durability.
Contact Our Technical Experts Today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Piotr Siwak, Roman Gr. Maev. The CaO Enhanced Defluorination and Air-Jet Separation of Cathode-Active Material Coating for Direct Recycling Li-Ion Battery Electrodes. DOI: 10.3390/met14121466
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the impact of microwave power on the synthesis of 2D metal oxides? Master High-Speed Material Production
- What are the complexities and maintenance requirements of continuous furnaces? Optimize High-Volume Production with Expert Insights
- How does the lab oven drying process ensure the quality of bimetallic catalysts? Master Pore Stability & Dispersion
- How does the pre-oxidation process affect high-temperature alloys? Enhancing Surface Integrity for Steam Cracking
- What role does a high-temperature curing oven play in lignin-modified wood? Unlock Superior Dimensional Stability
- Why is a heating furnace with high-precision temperature control required for alpha-Fe2O3/FeOOH? Expert Synthesis Guide
- What is the function of the 1500 °C environment in wood carbonization? Unlock High-Performance Functional Carbon
- Why is it necessary to use an annealing furnace at 350°C for three hours? Ensuring Glass Stability and Clarity



















