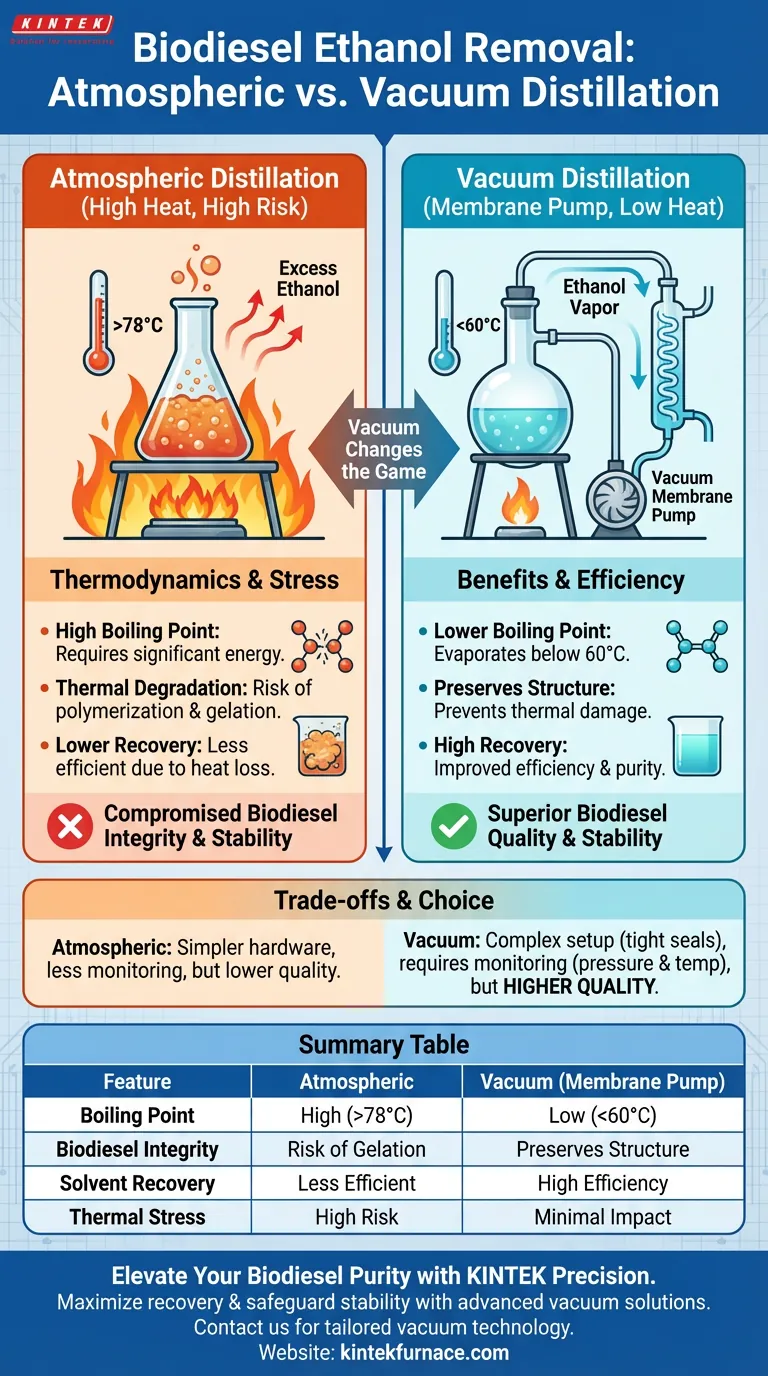
Vacuum distillation is the superior method for ethanol removal because it fundamentally alters the physical conditions of the separation process. By utilizing a vacuum membrane pump to reduce pressure, you significantly lower the boiling point of ethanol, allowing distillation to occur at much cooler temperatures—specifically below 60 degrees Celsius. This protects the biodiesel from the high-heat environment that characterizes atmospheric distillation.
The core advantage of vacuum distillation is the preservation of your biodiesel's chemical structure. By removing ethanol at reduced temperatures, you prevent the thermal degradation and gelation of fatty acid ethyl esters, ensuring a stable, high-quality final product.
The Thermodynamics of Ethanol Removal
Lowering the Boiling Point
Under atmospheric pressure, removing excess ethanol requires substantial heat to reach its natural boiling point.
By introducing a vacuum membrane pump, you create a reduced-pressure environment. This allows ethanol to vaporize at significantly lower temperatures, often below 60 degrees Celsius.
Improving Recovery Efficiency
Lowering the temperature does not mean slowing the process down.
Vacuum environments often speed up the rate of evaporation relative to the energy input. This leads to improved ethanol recovery efficiency, allowing you to reclaim and reuse more solvent with less thermal stress on the system.
Preserving Chemical Integrity
Preventing Polymerization
Biodiesel contains fatty acid ethyl esters, which are sensitive to high temperatures.
When exposed to the heat required for atmospheric distillation, these esters can undergo polymerization. This chemical reaction links molecules together, negatively altering the viscosity and flow properties of your fuel.
Avoiding Gelation and Decomposition
The presence of alkaline residues in the biodiesel mixture creates a specific vulnerability during heating.
High heat combined with these residues often triggers thermal decomposition or gelation. Vacuum distillation bypasses this risk entirely by keeping the process temperature below the threshold where these reactions occur.
Maintaining Stability
The ultimate goal of production is a stable, consistent fuel.
By avoiding high-temperature reactions, you preserve the chemical stability of the final product. This ensures the biodiesel meets quality standards and does not degrade during storage.
Understanding the Trade-offs
Equipment Complexity
While vacuum distillation offers superior product quality, it introduces hardware complexity.
Using a vacuum membrane pump requires a tighter seal on your system compared to atmospheric setups. You must ensure all connections are leak-proof to maintain the necessary reduced pressure.
operational Monitoring
Vacuum systems require more vigilant monitoring than simple boiling setups.
Operators must track both temperature and pressure simultaneously to prevent "bumping" (sudden boiling) or solvent loss into the pump.
Making the Right Choice for Your Production
To select the best method for your biodiesel facility, consider your quality and efficiency targets.
- If your primary focus is product quality: Rely on vacuum distillation to eliminate the risks of gelation and polymerization caused by high heat.
- If your primary focus is solvent reuse: Implement the vacuum method to maximize ethanol recovery while keeping the solvent chemically pure for future batches.
Vacuum distillation transforms a potentially destructive heating process into a controlled, efficient step that safeguards your fuel's integrity.
Summary Table:
| Feature | Atmospheric Distillation | Vacuum Distillation (Membrane Pump) |
|---|---|---|
| Boiling Point | High (Ethanol @ 78°C+) | Low (Below 60°C) |
| Biodiesel Integrity | Risk of polymerization/gelation | Preserves chemical structure |
| Solvent Recovery | Less efficient due to heat loss | High efficiency & purity |
| Thermal Stress | High risk of decomposition | Minimal thermal impact |
| Process Control | Basic temperature monitoring | Dual pressure & temperature control |
Elevate Your Biodiesel Purity with KINTEK Precision
Maximize your ethanol recovery and safeguard your biodiesel's chemical stability with our advanced vacuum solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems and lab high-temp furnaces—including Muffle, Tube, Rotary, and CVD systems—all fully customizable to your specific production needs.
Don't compromise on fuel quality. Contact KINTEK today to discover how our tailored vacuum technology can optimize your distillation workflow.
Visual Guide
References
- Sofia M. Kosolapova, Viacheslav A. Rudko. The Physicochemical Basis for the Production of Rapeseed Oil Fatty Acid Esters in a Plug Flow Reactor. DOI: 10.3390/pr12040788
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What type of reaction environment is required for the synthesis of Ge-Se-Tl-Sb chalcogenide glasses? | KINTEK
- Why are different cooling methods compared for GFRP post-fire performance? Evaluate Thermal Shock & Safety Risks
- What are the primary advantages of using powder metallurgy for Ti and TiZr alloys? Achieve Ultimate Structural Precision
- What role does a high-pressure autoclave play in the synthesis of the (NiZnMg)MoN precursor? Achieve Structural Precision
- Why is the vacuum drying process essential for the synthesis of phthalonitrile-modified titanium dioxide? Expert Guide
- What is the operating principle of a vacuum freeze-dryer in the fabrication of carbon aerogels? Master Sublimation
- What is the role of a laboratory drying oven in catalyst precursor control? Maximize Dispersion and Stability
- Why is Magnesium Hydride (MgH2) preferred for SiOx pre-magnesiation? Optimize Thermal Control and Battery Stability



















