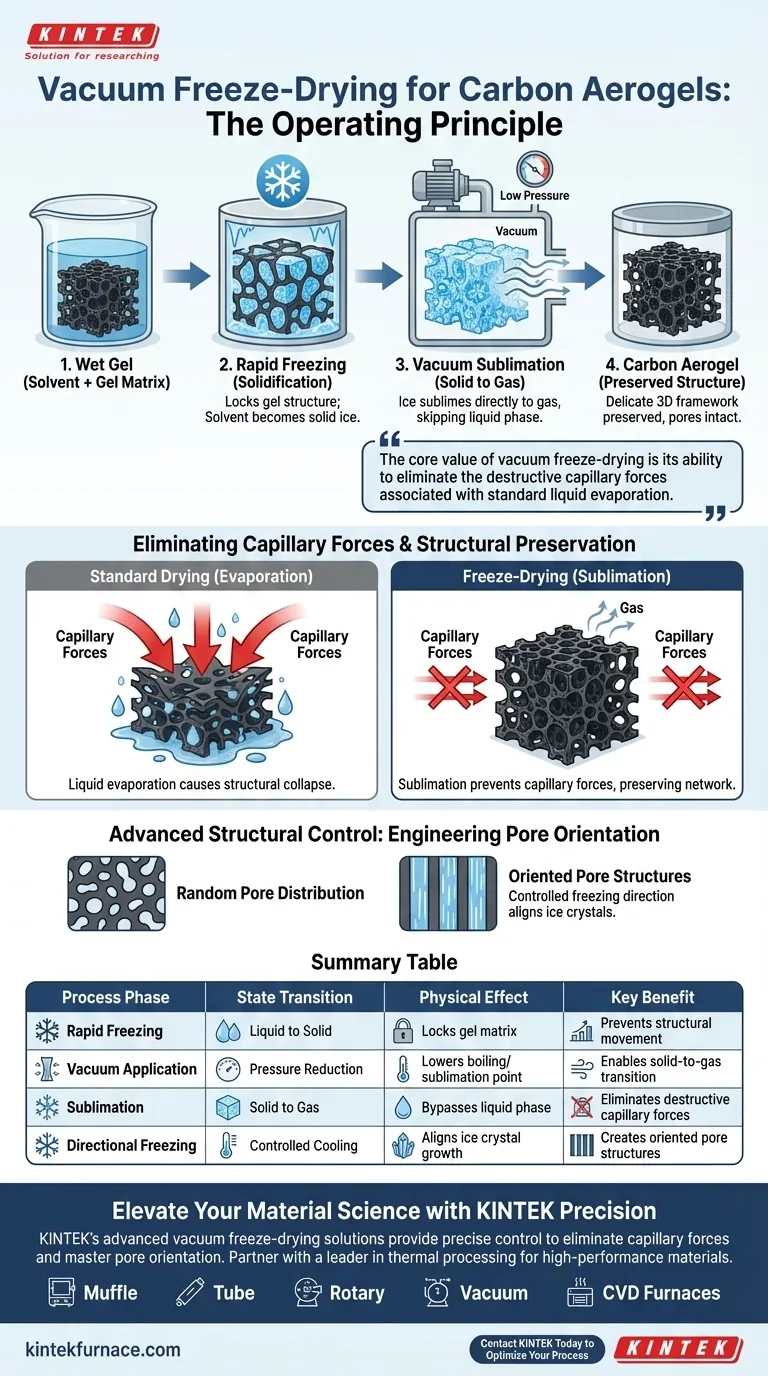
The operating principle of a vacuum freeze-dryer centers on the physical process of sublimation to remove solvents from a gel without damaging its structure. By rapidly freezing the solvent—typically water or tert-butanol—and subjecting it to a vacuum, the equipment converts the solid ice directly into gas, bypassing the liquid phase entirely.
The core value of vacuum freeze-drying is its ability to eliminate the destructive capillary forces associated with standard liquid evaporation. This preserves the aerogel's delicate three-dimensional framework and allows for the engineering of specific pore structures.

The Mechanics of Structural Preservation
The Role of Rapid Freezing
The process begins by stabilizing the wet gel. The freeze-dryer rapidly freezes the solvent contained within the gel matrix into a solid state.
Common solvents used in this phase include water or tert-butanol. This step effectively locks the physical structure of the gel in place, preparing it for solvent removal.
Understanding Sublimation
Once the solvent is frozen, the environment is shifted to vacuum conditions.
Under this low pressure, the solid solvent undergoes sublimation. This means it transitions directly from a solid to a gas, completely skipping the liquid phase.
Eliminating Capillary Forces
The primary engineering challenge in aerogel fabrication is maintaining the material's structural integrity.
Standard drying methods involve liquid evaporation, which generates significant capillary forces. These forces often crush the delicate three-dimensional framework of the gel.
By utilizing sublimation, the freeze-dryer prevents these capillary forces from forming, ensuring the internal network remains intact.
Advanced Structural Control
Engineering Pore Orientation
Beyond simple preservation, the freeze-dryer serves as a tool for structural engineering.
By precisely adjusting the freezing direction during the initial phase, the process can induce the formation of oriented pore structures.
This allows manufacturers to tailor the internal architecture of the carbon aerogel for specific applications rather than relying on random pore distribution.
Common Pitfalls to Avoid
The Risk of Liquid Re-formation
The success of this process relies entirely on maintaining the solvent in a solid state until it becomes gas.
If the vacuum pressure is insufficient or temperature controls fluctuate, the solvent may melt back into a liquid.
Structural Collapse
Once the solvent returns to a liquid state, capillary forces immediately re-engage.
This will lead to the collapse of the three-dimensional framework, rendering the aerogel useless. Strict adherence to sublimation parameters is required to prevent this failure mode.
Optimizing the Fabrication Process
To ensure the highest quality carbon aerogels, align your process parameters with your specific structural goals:
- If your primary focus is Maximum Structural Integrity: Ensure the vacuum level is sufficient to maintain pure sublimation, preventing any liquid phase that would trigger capillary collapse.
- If your primary focus is Directed Transport Properties: Actively control the freezing direction during the initial cooling phase to create aligned, oriented pore structures.
Mastering the transition from solid to gas is the key to producing robust, high-performance aerogels.
Summary Table:
| Process Phase | State Transition | Physical Effect | Key Benefit |
|---|---|---|---|
| Rapid Freezing | Liquid to Solid | Locks gel matrix in place | Prevents structural movement |
| Vacuum Application | Pressure Reduction | Lowers boiling/sublimation point | Enables solid-to-gas transition |
| Sublimation | Solid to Gas | Bypasses liquid phase | Eliminates destructive capillary forces |
| Directional Freezing | Controlled Cooling | Aligns ice crystal growth | Creates oriented pore structures |
Elevate Your Material Science with KINTEK Precision
Are you struggling with structural collapse during carbon aerogel fabrication? KINTEK’s advanced vacuum freeze-drying solutions are engineered to provide the precise temperature and pressure control necessary to eliminate capillary forces and master pore orientation.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab high-temp systems, including Muffle, Tube, Rotary, Vacuum, and CVD furnaces, all fully customizable to meet your unique research or production requirements. Partner with a leader in thermal processing to ensure the integrity of your high-performance materials.
Contact KINTEK Today to Optimize Your Process
Visual Guide
References
- Yong Zhong, Xuguang Liu. Carbon Aerogel for Aqueous Phase Adsorption/Absorption: Application Performances, Intrinsic Characteristics, and Regulatory Constructions. DOI: 10.1002/sstr.202400650
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What role does a controlled curing environment play for refractory castables? Ensure Structural Integrity & Precision
- What role does a high-temperature heating environment play in the hydrothermal synthesis of ZSM-5 zeolite crystals?
- What are the limitations of functional group grafting through high-temperature heating? Achieve Chemical Precision
- Why use 10% Carbon Monoxide in black liquor pyrolysis? Prevent sodium volatilization for superior char quality.
- Why is calcination at 700 °C necessary for extracted diatomaceous biosilica? Achieve Peak Material Stability
- How does a laboratory oven function during PDMS curing? Achieve Precision in Device Encapsulation
- Why must calcination equipment be used to pre-treat ZSM-5 zeolite? Ensure Accurate VOC Adsorption Testing
- How does an annealing furnace work? A Guide to Controlled Heat Treatment



















