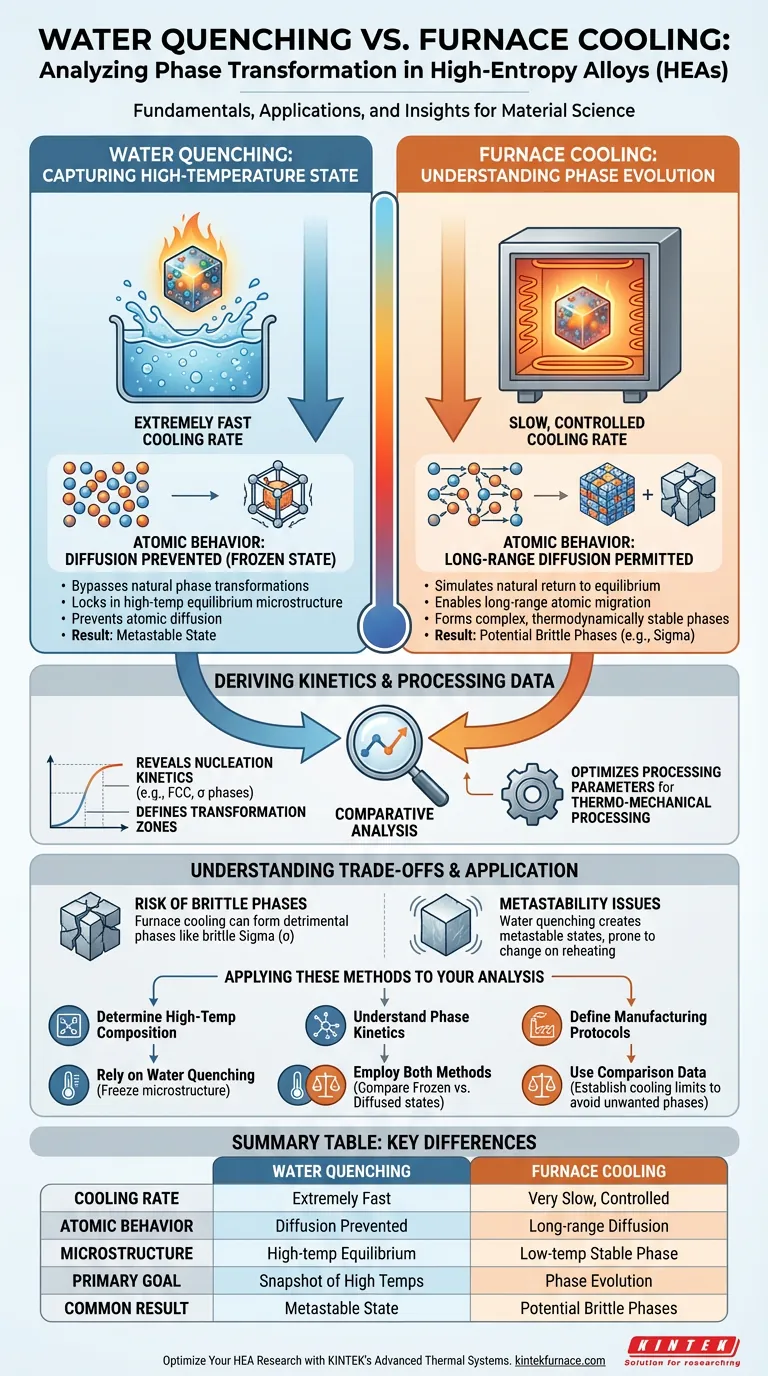
Water quenching and furnace cooling differ fundamentally in their cooling rates and the resulting atomic behavior within high-entropy alloys. Water quenching is a rapid process designed to "freeze" the alloy's high-temperature state instantly, preventing structural changes. In contrast, furnace cooling is a slow, controlled process that allows ample time for atoms to migrate and rearrange, resulting in a microstructure that reflects the alloy's natural evolution during cooling.
By comparing the results of these two distinct methods, researchers can identify the nucleation kinetics of specific phases and determine the optimal cooling parameters required for effective thermo-mechanical processing.

Capturing the High-Temperature State
The Mechanics of Water Quenching
Water quenching utilizes an extremely fast cooling rate. This rapid drop in temperature is intended to bypass the time required for natural phase transformations to occur.
Preserving Equilibrium Microstructures
The primary goal of this method is to lock in the high-temperature equilibrium microstructure. By bringing the alloy to room temperature instantly, researchers capture a "snapshot" of the material as it existed in the furnace.
preventing Atomic Diffusion
Because the cooling is instantaneous, atoms do not have time to move. This effectively allows for the precise observation of phase compositions exactly as they exist at elevated temperatures.
Understanding Phase Evolution
The Role of Slow Cooling
Furnace cooling provides a significantly different environment characterized by a gradual reduction in temperature. This simulates a more natural return to equilibrium conditions.
Enabling Long-Range Diffusion
Unlike quenching, furnace cooling permits long-range atomic diffusion. The extended time at elevated temperatures allows atoms to migrate across the material lattice.
Forming Complex Phases
This diffusion facilitates the formation of thermodynamically stable phases. It reveals how the material prefers to arrange itself when given the opportunity to reach low-temperature equilibrium.
Deriving Kinetics and Processing Data
Revealing Nucleation Kinetics
Comparing the "frozen" state of a quenched sample against the "evolved" state of a furnace-cooled sample provides critical data. This comparison highlights the nucleation kinetics of specific phases, such as Face-Centered Cubic (FCC) and sigma (σ) phases.
Defining Transformation Zones
By analyzing the differences, researchers can identify exactly where and how phase transformations occur as the alloy passes through specific transformation zones.
Optimizing Processing Parameters
This comparative analysis is essential for manufacturing. It helps engineers define the precise cooling parameters needed for successful thermo-mechanical processing, ensuring the final material has the desired properties.
Understanding the Trade-offs
The Risk of Brittle Phases
While furnace cooling reveals equilibrium states, it often allows the formation of detrimental phases. For example, allowing full diffusion can lead to the precipitation of the sigma (σ) phase, which is often brittle and may degrade the alloy's performance.
Metastability Issues
Conversely, water quenching creates a metastable state. While it preserves the high-temperature structure, the resulting material may be thermodynamically unstable and prone to changing if reheated or stressed.
Applying These Methods to Your Analysis
To determine the correct approach for your high-entropy alloy project, consider your specific analytical goals:
- If your primary focus is determining high-temperature composition: Rely on water quenching to freeze the microstructure and eliminate diffusion artifacts.
- If your primary focus is understanding phase kinetics: You must employ both methods to compare the "frozen" state against the "diffused" state to map nucleation zones.
- If your primary focus is defining manufacturing protocols: Use the comparison data to establish cooling limits that avoid the formation of unwanted phases like sigma (σ) during thermo-mechanical processing.
Mastering the contrast between these cooling rates is the key to controlling the final microstructure and performance of your alloy.
Summary Table:
| Feature | Water Quenching | Furnace Cooling |
|---|---|---|
| Cooling Rate | Extremely Fast | Very Slow / Controlled |
| Atomic Behavior | Diffusion is prevented; "Frozen" state | Long-range diffusion permitted |
| Microstructure | High-temperature equilibrium state | Low-temperature stable phase |
| Primary Goal | Snapshot of elevated temperatures | Understanding phase evolution |
| Common Result | Metastable state | Potential brittle phase (e.g., sigma) |
Optimize Your HEA Research with KINTEK
Precision in phase transformation study requires exact thermal control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for advanced metallurgy. Whether you need rapid quenching or controlled furnace cooling, our customizable lab high-temperature furnaces provide the reliability your research demands.
Ready to refine your material properties? Contact KINTEK today for a customized solution!
Visual Guide
References
- Mudassar Hussain, Tuty Asma Abu Bakar. X-Ray Diffraction Analysis of Sigma-Phase Evolution in Equimolar AlCoCrFeNi High Entropy Alloy. DOI: 10.15282/ijame.21.4.2024.14.0917
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering and Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the primary purpose of using a laboratory constant temperature drying oven for fuel sample preparation?
- What causes large-scale MgO·Al2O3 inclusions in superalloys? Expert Guide to Formation & Prevention
- Why is an air-ventilated oven necessary for GFPP surface modification? Achieve Maximum Solar Reflectance
- What is the purpose of the annealing process in OLED preparation? Optimize Film Stability and Device Efficiency
- Why is a 1:1 mixture of NaNO3 and KNO3 used in molten salt baths? Optimize Quenching Performance
- What happens during the sintering process? Transform Powder into Dense, High-Strength Components
- How does a constant temperature forced air drying oven contribute to the pore activation process of biomass carbon?
- Why is it necessary to dry glassware in a 140 °C oven overnight before GTP? Ensure Precise Anhydrous Polymerization










