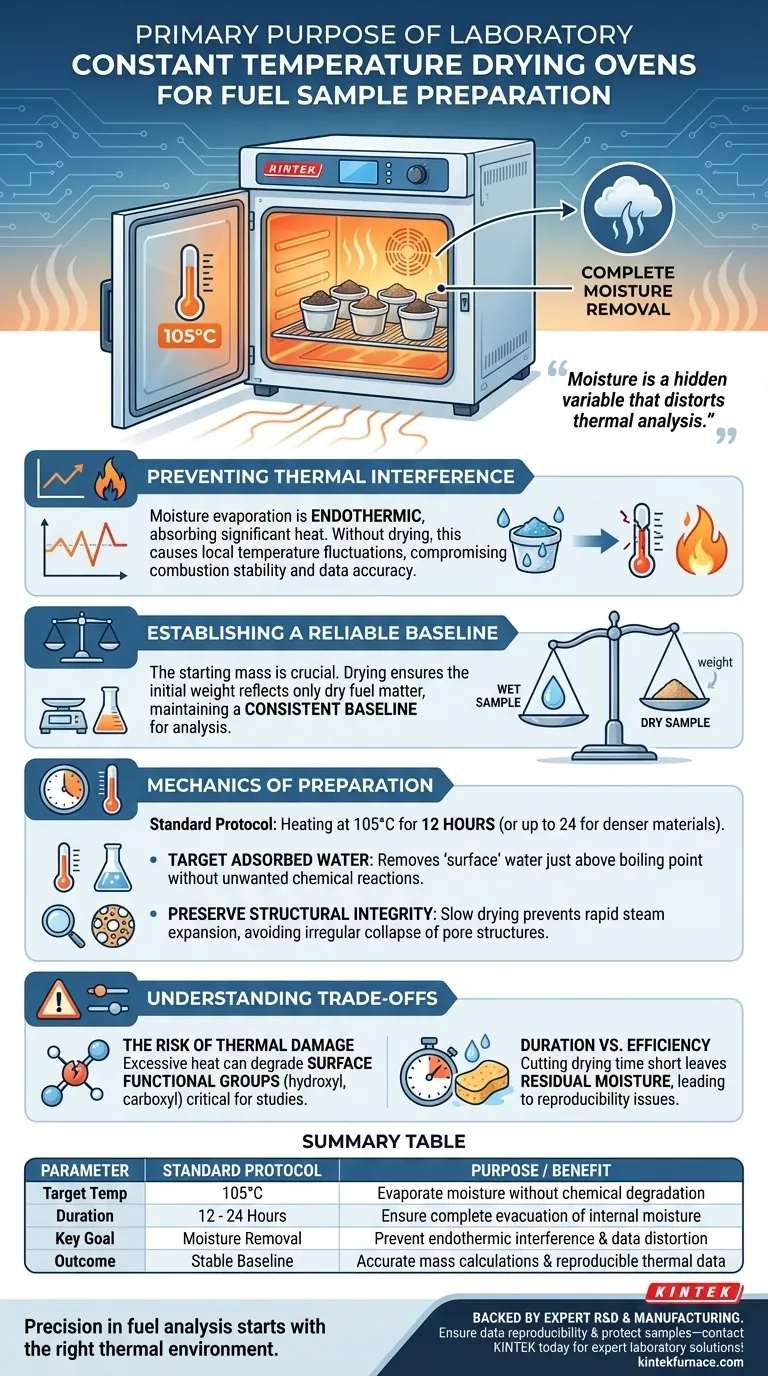
The primary purpose of using a laboratory constant temperature drying oven for fuel sample preparation is to completely remove physically adsorbed moisture. By treating samples at a controlled temperature (typically 105°C) for a set duration, you prevent moisture-induced endothermic effects during subsequent high-temperature experiments, ensuring combustion stability and data reproducibility.
Moisture is a hidden variable that distorts thermal analysis. By eliminating it beforehand, you ensure that any thermal changes observed during experimentation are caused by the fuel's chemical properties, not by the energy-consuming process of water evaporation.

The Critical Role of Moisture Removal
Preventing Thermal Interference
When fuel samples containing moisture are introduced into high-temperature environments, such as a tube furnace, the water must evaporate before the fuel can react.
This evaporation process is endothermic, meaning it absorbs significant heat from the immediate environment.
Without prior drying, this heat absorption causes local temperature fluctuations, interfering with the stability of the combustion temperature and compromising the accuracy of the experiment.
Establishing a Reliable Baseline
For analytical techniques like elemental analysis or thermogravimetric analysis (TGA), the starting mass of the sample is the foundation of all calculations.
If physically adsorbed water remains in the sample, the initial weight reading will be artificially high.
The drying oven ensures that the "starting weight" reflects only the dry fuel matter, maintaining a consistent baseline across all tested samples.
Mechanics of Preparation
Targeting Adsorbed Water
The standard protocol for fuel samples involves heating at 105°C for 12 hours.
This specific temperature is chosen because it is just above the boiling point of water, sufficient to drive off free moisture without triggering unwanted chemical reactions in the fuel itself.
This effectively removes the "surface" water that the sample has absorbed from the ambient atmosphere.
Preserving Structural Integrity
Rapid heating of wet samples during high-temperature pyrolysis can cause internal water to flash into steam instantaneously.
This rapid expansion can rupture the internal structure of the material, leading to irregular collapse of pore structures.
Slow, constant drying at lower temperatures prevents this physical damage, ensuring the physical characteristics of the fuel or biochar remain intact for analysis.
Understanding the Trade-offs
The Risk of Thermal Damage
While removing moisture is essential, setting the oven temperature too high can act counterproductively.
Excessive heat can degrade surface functional groups, such as hydroxyl and carboxyl groups, which are often critical for adsorption studies.
Precise temperature control is necessary to dry the sample without destroying the active sites or altering the chemical composition before the actual experiment begins.
Duration vs. Efficiency
There is a balance between drying speed and thoroughness.
While fuel samples often require 12 hours, denser materials like oil shale or coconut husk may require up to 24 hours to ensure internal moisture is fully evacuated.
Cutting this time short leaves residual moisture deep within the material structure, leading to the very data reproducibility issues the process is meant to solve.
Making the Right Choice for Your Goal
To apply this correctly, align your drying protocol with your specific experimental needs:
- If your primary focus is Thermal Stability: Ensure the sample is dried at 105°C to prevent endothermic reactions from destabilizing your furnace temperature.
- If your primary focus is Surface Chemistry: Consider lower drying temperatures (e.g., 50°C) or careful monitoring to prevent the thermal degradation of sensitive functional groups.
A disciplined drying protocol is the invisible step that transforms raw data into reproducible science.
Summary Table:
| Parameter | Standard Protocol | Purpose / Benefit |
|---|---|---|
| Target Temp | 105°C | Evaporate moisture without chemical degradation |
| Duration | 12 - 24 Hours | Ensure complete evacuation of internal moisture |
| Key Goal | Moisture Removal | Prevent endothermic interference & data distortion |
| Outcome | Stable Baseline | Accurate mass calculations & reproducible thermal data |
Precision in fuel analysis starts with the right thermal environment. Backed by expert R&D and manufacturing, KINTEK offers a wide range of high-performance lab equipment including Muffle, Tube, Rotary, and Vacuum systems, alongside customizable constant temperature drying ovens designed for your unique research needs. Ensure your data reproducibility and protect your samples from thermal interference—contact KINTEK today for expert laboratory solutions!
Visual Guide
References
- Silin Zeng, Baosheng Jin. Experimental study on No<sub>x</sub> emission and nitrogen conversion characteristics of a coal gangue blended with coal washing mixture. DOI: 10.1088/1742-6596/3013/1/012035
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- What role does a high-frequency LCR meter play in analyzing the CIS of SSBSN ceramics? Unlocking Microstructural Secrets
- Why is programmable temperature control in furnaces critical during superalloy aluminization? Ensure High-Yield HTLA
- What are the three types of dental ceramics? A Guide to Material Selection
- What is the function of injecting water in wood thermal modification? Unlock Superior Stability and Hydrophobicity
- What is the significance of using a laboratory vacuum drying oven during the catalyst recovery phase of depolymerization?
- How do chill rings specifically influence the temperature field distribution? Expert Insight into Crystal Casting
- Why must Ru/GNK catalysts undergo vacuum drying? Ensure Peak Performance with Safe Desorption
- What is the purpose of high-vacuum thermal evaporation coating equipment in SiQD LED fabrication? Expert Insights



















