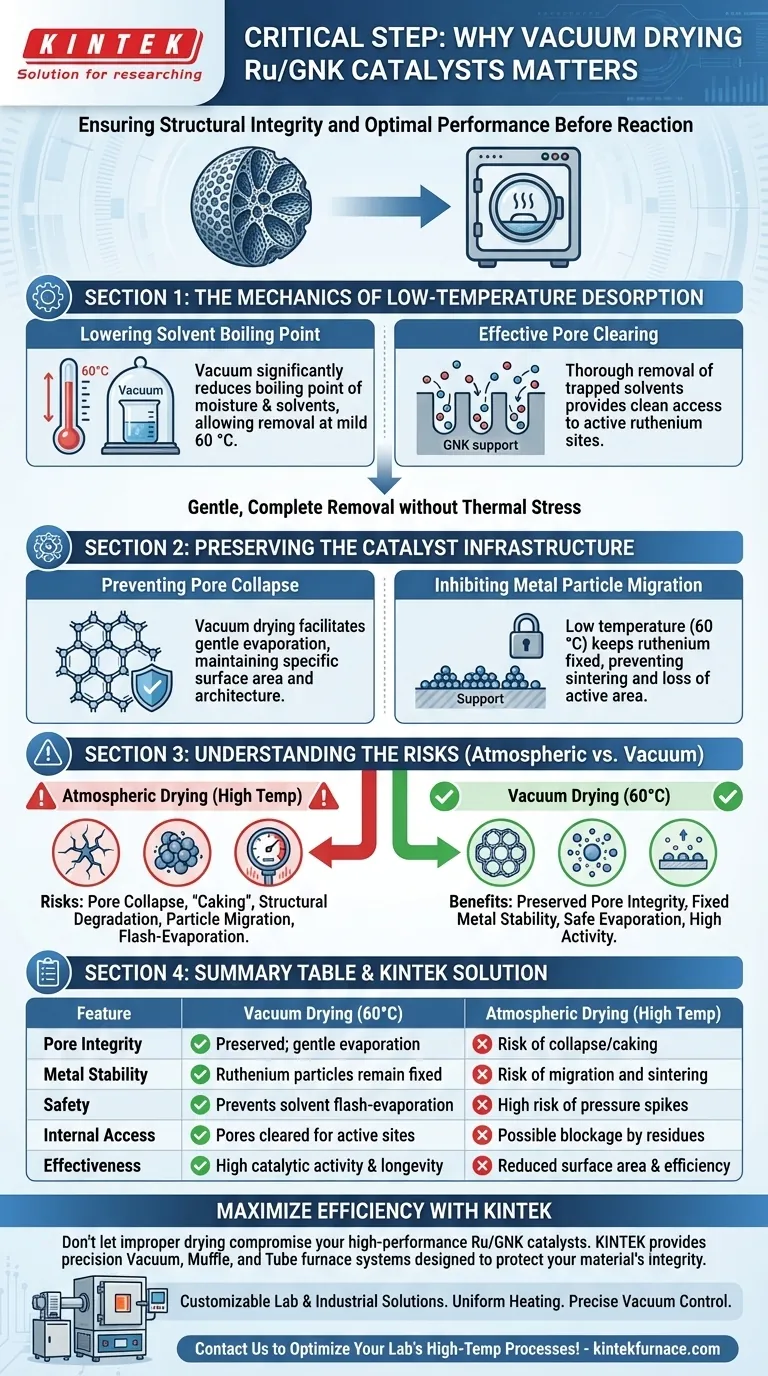
Vacuum drying Ru/GNK catalysts is a critical preparation step used to remove residual moisture and solvents while preserving the catalyst's structural integrity. By utilizing a vacuum at 60 °C, researchers can effectively clear the catalyst's internal pores without subjecting the material to the damaging thermal stress associated with high-temperature atmospheric drying.
Vacuum drying protects the catalyst's "as-prepared" state by lowering solvent boiling points, which prevents the collapse of the support pores and stops ruthenium particles from migrating or aggregating.

The Mechanics of Low-Temperature Desorption
Lowering the Solvent Boiling Point
The primary function of the vacuum environment is to significantly reduce the boiling point of trapped moisture and solvents.
This allows for the thorough removal of liquids at a mild temperature of 60 °C, which would otherwise require much higher heat under standard atmospheric pressure.
Effective Pore Clearing
Residual solvents trapped deep within the GNK (Graphitized Nano-Knitted) support can interfere with the subsequent reaction if not removed.
Vacuum drying ensures these pores are emptied completely, providing a clean surface area for the reactant molecules to access the active ruthenium sites.
Preserving the Catalyst Infrastructure
Preventing Pore Collapse
High-temperature drying at atmospheric pressure can cause rapid evaporation and internal pressure changes that lead to the collapse of the support’s delicate pore structure.
Vacuum drying facilitates a gentler evaporation process, maintaining the specific surface area and architecture of the GNK support required for high catalytic activity.
Inhibiting Metal Particle Migration
Excessive heat during the drying phase often triggers the migration of metal particles across the support surface.
By keeping the temperature at 60 °C through vacuum assistance, the ruthenium particles remain fixed in their intended positions, preventing sintering and loss of active surface area.
Understanding the Trade-offs and Risks
Atmospheric vs. Vacuum Drying
Atmospheric drying is often faster and requires less specialized equipment, but it poses a high risk of "caking" or structural degradation.
While vacuum drying requires a dedicated oven and longer processing times to achieve a full vacuum, the resulting catalyst stability far outweighs these minor operational costs.
The Danger of Residual Solvents
If a catalyst is placed directly into a reaction furnace without vacuum drying, residual solvents may flash-evaporate at high temperatures.
This "flash" can cause physical fracturing of the catalyst particles and lead to unpredictable pressure spikes within the reaction vessel.
Ensuring Optimal Catalyst Performance
To achieve the best results with your Ru/GNK catalyst, drying parameters must be strictly controlled to balance efficiency with material safety.
- If your primary focus is maximizing catalyst longevity: Always utilize vacuum drying at 60 °C to ensure the pore structure remains intact for multiple reaction cycles.
- If your primary focus is preventing metal sintering: Maintain a consistent vacuum to keep drying temperatures low, ensuring ruthenium particles do not migrate and clump together.
By prioritizing this controlled drying phase, you ensure that the catalyst enters the reaction furnace in its most potent and stable form.
Summary Table:
| Feature | Vacuum Drying (60°C) | Atmospheric Drying (High Temp) |
|---|---|---|
| Pore Integrity | Preserved; gentle evaporation | Risk of collapse/caking |
| Metal Stability | Ruthenium particles remain fixed | Risk of migration and sintering |
| Safety | Prevents solvent flash-evaporation | High risk of pressure spikes |
| Internal Access | Pores cleared for active sites | Possible blockage by residues |
| Effectiveness | High catalytic activity & longevity | Reduced surface area & efficiency |
Maximize Your Catalyst Efficiency with KINTEK
Don’t let improper drying compromise your high-performance Ru/GNK catalysts. Backed by expert R&D and world-class manufacturing, KINTEK provides precision Vacuum, Muffle, and Tube furnace systems specifically designed to protect your material’s structural integrity.
Whether you need customizable lab furnaces or industrial-scale solutions, our equipment ensures uniform heating and precise vacuum control to prevent sintering and pore collapse. Contact us today to optimize your lab's high-temperature processes!
Visual Guide
References
- Mukesh Kumar, Sudhanshu Sharma. Natural kaolin-derived ruthenium-supported nanoporous geopolymer: a sustainable catalyst for CO <sub>2</sub> methanation. DOI: 10.1039/d5cy00021a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does an infrared rapid thermal annealing belt furnace affect battery performance? Maximize Efficiency Today
- How does a laboratory vacuum drying oven contribute to the post-processing stage of pBN-CTF products?
- What core parameters does a sessile drop furnace provide for quartz glass? Master High-Temp Material Evaluation
- What is the technical necessity of heating and stirring for K-Na alloy anodes? Ensure Peak Battery Performance
- Why is a constant temperature blast drying oven necessary for biomass carbon impregnation? Optimize Material Structure
- What role does a laboratory oven play when coating nanocomposite powders? Master Thin Film Sensor Fabrication
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control
- What functions does glucose perform in lithium-ion sieve synthesis? Enhance Carbothermal Reduction for LiMnO2 Purity



















