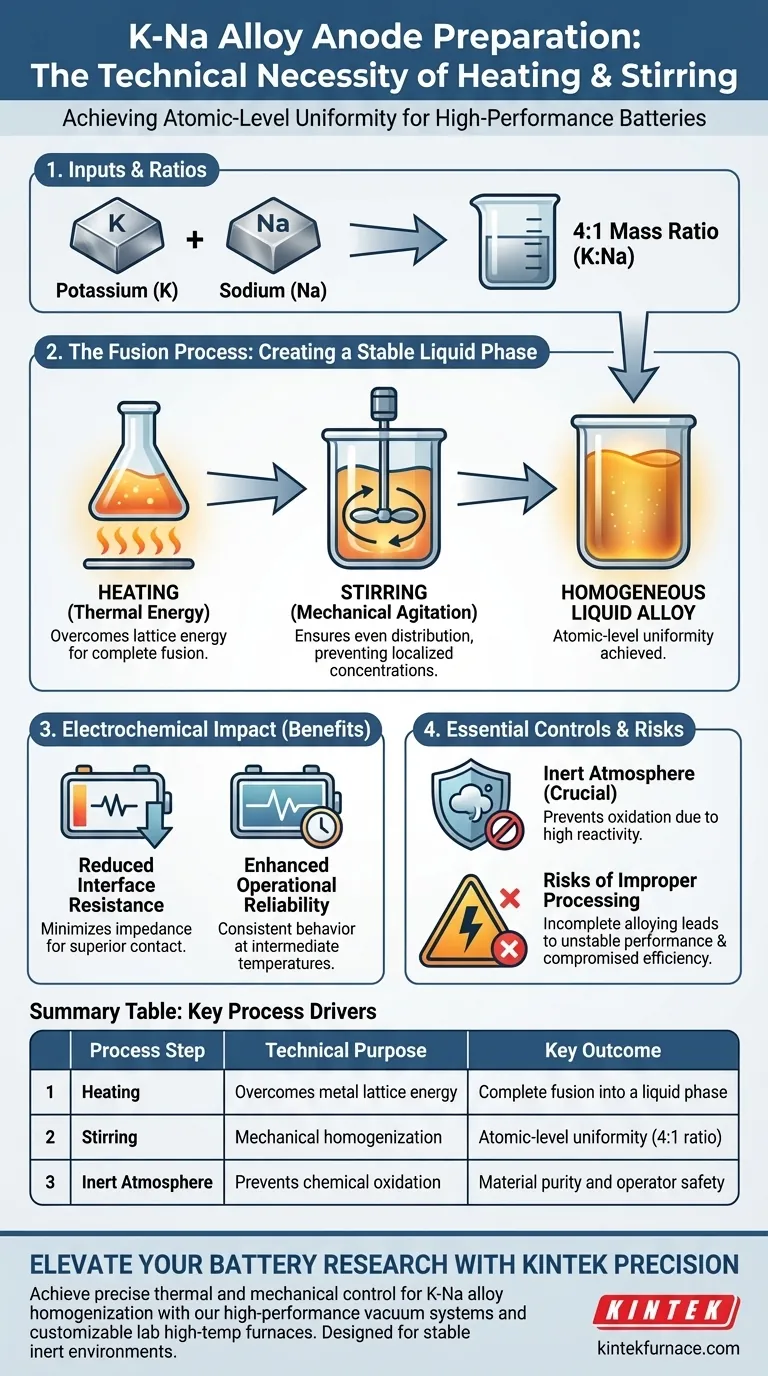
The technical necessity of heating and stirring lies in their ability to force high-purity metallic potassium and sodium into a single, homogeneous liquid phase. Without these active mechanical and thermal inputs, the metals cannot achieve the atomic-level uniformity required to function effectively as a battery anode.
Core Takeaway The combination of heating and stirring is not merely for mixing; it is a fusion process required to create a stable liquid alloy from distinct metals. This precise homogenization is the primary factor in lowering interface resistance and ensuring the battery’s reliability during intermediate-temperature operation.

Creating a Stable Liquid Phase
Achieving Complete Fusion
Simply placing potassium and sodium in contact is insufficient for creating a functional anode. Heating provides the necessary thermal energy to overcome the lattice energy of the individual metals, allowing them to fuse completely.
The Role of Mechanical Agitation
Stirring acts as the catalyst for uniformity. It ensures that the potassium and sodium atoms are distributed evenly throughout the volume of the material, preventing localized concentrations of either pure metal.
Adhering to Precise Ratios
The standard protocol typically requires a 4:1 mass ratio of potassium to sodium. Maintaining this specific ratio through proper mixing techniques is essential for the alloy to reach its intended stable liquid state.
Impact on Electrochemical Performance
Reducing Interface Resistance
The primary electrochemical goal of this process is to minimize impedance. A thoroughly mixed, liquid K-Na alloy creates a superior contact interface, significantly reducing interface resistance within the cell.
Enhancing Operational Reliability
Batteries operating at intermediate temperatures require consistent anode behavior. The homogeneity achieved through heating and stirring prevents performance fluctuations, thereby improving the long-term reliability of the system.
Essential Environmental Controls
Managing Reactivity
Potassium and sodium are highly reactive, particularly when heated. Therefore, the heating and stirring process must be conducted strictly under an inert atmosphere to prevent oxidation and ensure safety.
Risks of Improper Processing
Consequences of Incomplete Alloying
If the heating is insufficient or the stirring inadequate, the alloy will lack uniformity. This leads to chemically distinct regions within the anode, which causes unstable electrochemical performance.
Compromised Battery Efficiency
A poorly alloyed anode creates higher resistance pathways. This directly degrades the battery's efficiency and can lead to failure during intermediate-temperature operations.
Optimizing Anode Preparation
To ensure the production of high-performance K-Na anodes, consider the following based on your specific objectives:
- If your primary focus is electrochemical efficiency: Prioritize vigorous stirring and precise temperature control to minimize interface resistance.
- If your primary focus is material stability: Ensure strict adherence to the 4:1 mass ratio under a controlled inert atmosphere to prevent contamination.
Ultimately, the rigor applied to the heating and stirring process defines the ceiling of your battery's reliability and performance.
Summary Table:
| Process Step | Technical Purpose | Key Outcome |
|---|---|---|
| Heating | Overcomes metal lattice energy | Complete fusion into a liquid phase |
| Stirring | Mechanical homogenization | Atomic-level uniformity (4:1 mass ratio) |
| Inert Atmosphere | Prevents chemical oxidation | Material purity and operator safety |
| Homogenization | Minimizes impedance | Reduced interface resistance and stability |
Elevate Your Battery Research with KINTEK Precision
To achieve the precise thermal and mechanical control required for K-Na alloy homogenization, you need laboratory equipment engineered for excellence. Backed by expert R&D and manufacturing, KINTEK offers high-performance vacuum systems and customizable lab high-temp furnaces designed to maintain stable inert environments and uniform heat distribution. Whether your project requires standard muffle furnaces or specialized CVD systems, our solutions are tailored to meet your unique materials science needs.
Ready to optimize your electrochemical performance? Contact KINTEK today to discover how our customizable heating solutions can enhance your lab’s efficiency.
Visual Guide
References
- Liying Tian, Zhichuan J. Xu. Dual Roles of Deep Eutectic Solvent in Polysulfide Redox and Catalysis for Intermediate‐Temperature Potassium‐Sulfur Batteries. DOI: 10.1002/adma.202507114
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What role does an industrial oven play in the pretreatment of oil palm shell for biochar? Ensure Peak Biomass Quality
- What is the objective of setting temperature gradients of 40 °C, 50 °C, and 60 °C? Optimize Yogurt Drying Viability
- How is a constant temperature drying oven utilized to establish moisture content gradients in wood? Master the Baseline
- Why is a precision electric heating reactor used for ozone treatment of porous graphene? Unlock Angstrom-Scale Accuracy
- Why must raw kaolin undergo heat treatment for DLP 3D printing? Control Viscosity for Precision Printing
- How does an infrared rapid thermal annealing belt furnace affect battery performance? Maximize Efficiency Today
- What is a laboratory furnace and why is it important? Unlock Precision Heating for Your Lab
- What is the function of a fixed-bed catalytic reactor in ex situ CHP? Optimize Your Bio-oil Quality Today



















