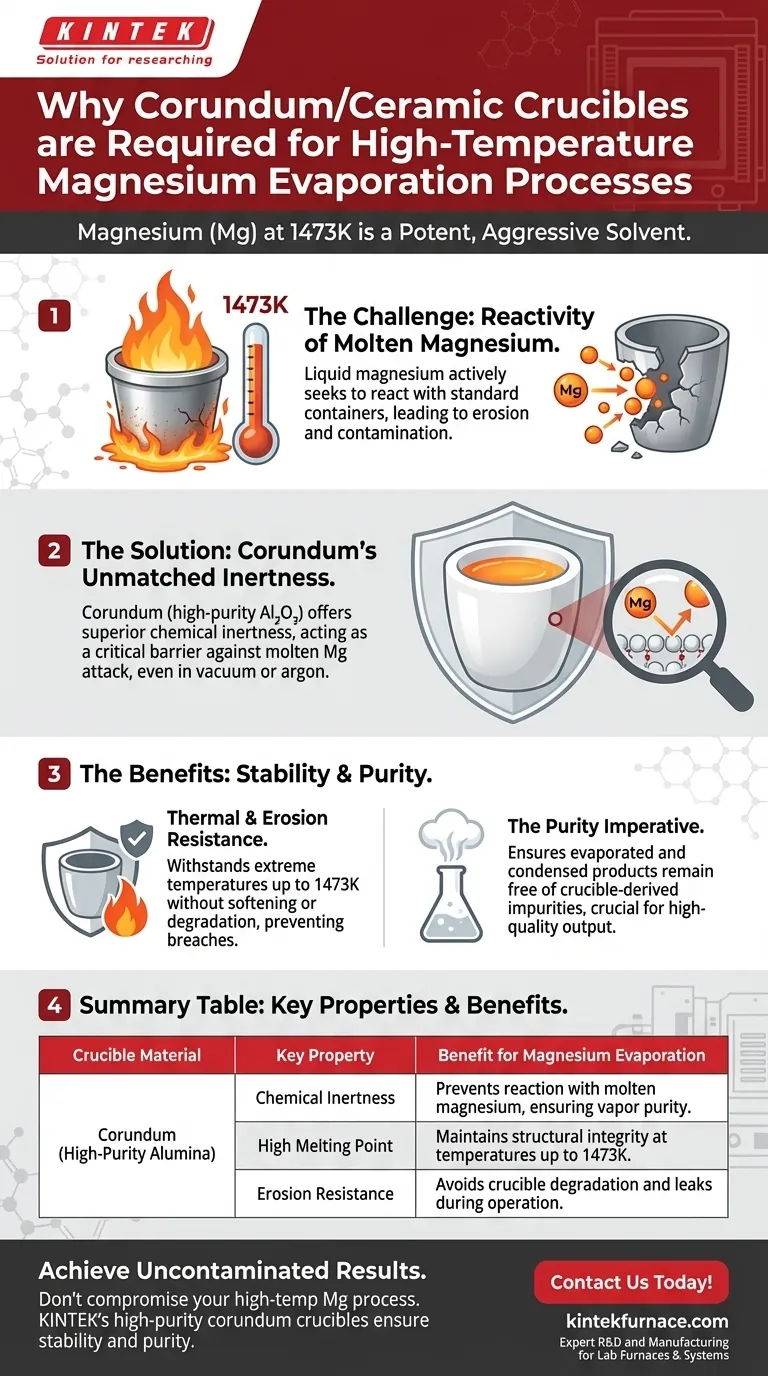
Corundum or ceramic crucibles are required because liquid magnesium acts as a potent solvent that aggressively attacks most standard container materials. At evaporation temperatures reaching 1473K, magnesium becomes chemically active, necessitating a vessel with superior inertness and erosion resistance. Corundum (high-purity aluminum oxide) provides this structural stability, preventing the crucible from degrading and ensuring the resulting magnesium vapor remains uncontaminated.
The primary driver for selecting corundum is chemical neutrality. While molten magnesium reacts with many metals and standard refractory materials, high-purity alumina remains inert, acting as a critical barrier that preserves the purity of the evaporation process.

The Chemistry of High-Temperature Magnesium
The Reactivity of Molten Magnesium
Liquid magnesium is not merely hot; it is chemically aggressive.
When heated to evaporation temperatures, magnesium actively seeks to react with the material holding it. This high reactivity makes standard metallic or lower-grade refractory containers unsuitable for the process.
The Consequence of Material Interaction
If a crucible reacts with the magnesium, two failures occur simultaneously: the crucible erodes, and the magnesium becomes contaminated.
This reaction introduces foreign elements into the melt, compromising the integrity of the final magnesium powder or condensate.
Why Corundum serves as the Ideal Interface
Unmatched Chemical Stability
Corundum, specifically high-purity alumina, possesses a unique resistance to chemical attack.
It serves as an inert container that refuses to bond with molten metallic magnesium. This inertness holds true even under the rigorous conditions of high vacuum or argon atmospheres used in these experiments.
Withstanding Extreme Thermal Stress
Magnesium evaporation often requires temperatures up to 1473K.
Corundum is selected because its melting point far exceeds this operational requirement. It maintains its structural rigidity without softening or chemically breaking down at temperatures where other materials would fail.
Understanding the Operational Trade-offs
The Necessity of Erosion Resistance
The choice of crucible is often a trade-off between cost and contamination control.
However, in magnesium evaporation, "erosion resistance" is not a luxury; it is a requirement. Using a lesser material results in physical degradation of the boat or crucible, potentially leading to breaches and leaks during the experiment.
The Purity Imperative
The ultimate trade-off involves the quality of your output.
If you utilize materials with lower chemical stability, you inevitably trade off the purity of your final product. Corundum ensures that the evaporated and condensed products remain free of crucible-derived impurities.
Making the Right Choice for Your Process
Selecting the correct vessel is determined by your tolerance for contamination and thermal failure.
- If your primary focus is Maximum Purity: You must use high-purity corundum or alumina crucibles to ensure zero chemical interaction with the molten magnesium.
- If your primary focus is Process Stability: Select corundum to prevent physical erosion and container failure at temperatures reaching 1473K.
By leveraging the inert properties of corundum, you transform a volatile chemical process into a controlled, high-yield operation.
Summary Table:
| Crucible Material | Key Property | Benefit for Magnesium Evaporation |
|---|---|---|
| Corundum (High-Purity Alumina) | Chemical Inertness | Prevents reaction with molten magnesium, ensuring vapor purity |
| Corundum (High-Purity Alumina) | High Melting Point | Maintains structural integrity at temperatures up to 1473K |
| Corundum (High-Purity Alumina) | Erosion Resistance | Avoids crucible degradation and leaks during operation |
Achieve Uncontaminated Results with the Right Crucible
Don't let crucible failure or contamination compromise your high-temperature magnesium evaporation process. At KINTEK, we understand the critical need for chemical inertness and thermal stability in demanding applications.
Our high-purity corundum and ceramic crucibles are engineered to withstand aggressive molten metals like magnesium, ensuring your process remains stable and your output pure. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for unique needs.
Contact us today to discuss how our solutions can enhance your lab's efficiency and reliability. Let's transform your volatile processes into controlled, high-yield operations.
Get in touch with our experts now!
Visual Guide
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the primary function of a high-purity alumina crucible in PrVSb3 synthesis? Ensure Chemical Inertness & Purity
- What technical considerations justify the use of high-purity alumina crucibles for microwave-assisted metal reduction?
- What role do laboratory furnaces play in quality control? Ensure Material Integrity and Product Reliability
- Why is a graphite crucible used for melting Al-Mg-Si alloys? Superior Purity & Thermal Efficiency
- Why are high-purity alumina crucibles preferred? Secure Unmatched Purity and Data Integrity in Lab Synthesis
- How does the design of a graphite box optimize the sulfurization of Sb thin films? Key Insights for Film Uniformity
- Why is a graphite crucible used and the melt temperature maintained at 750°C for AA7150-Al2O3? Optimize Your Composite
- What functions does a high-density graphite crucible perform? More Than a Container for Copper Refining



















