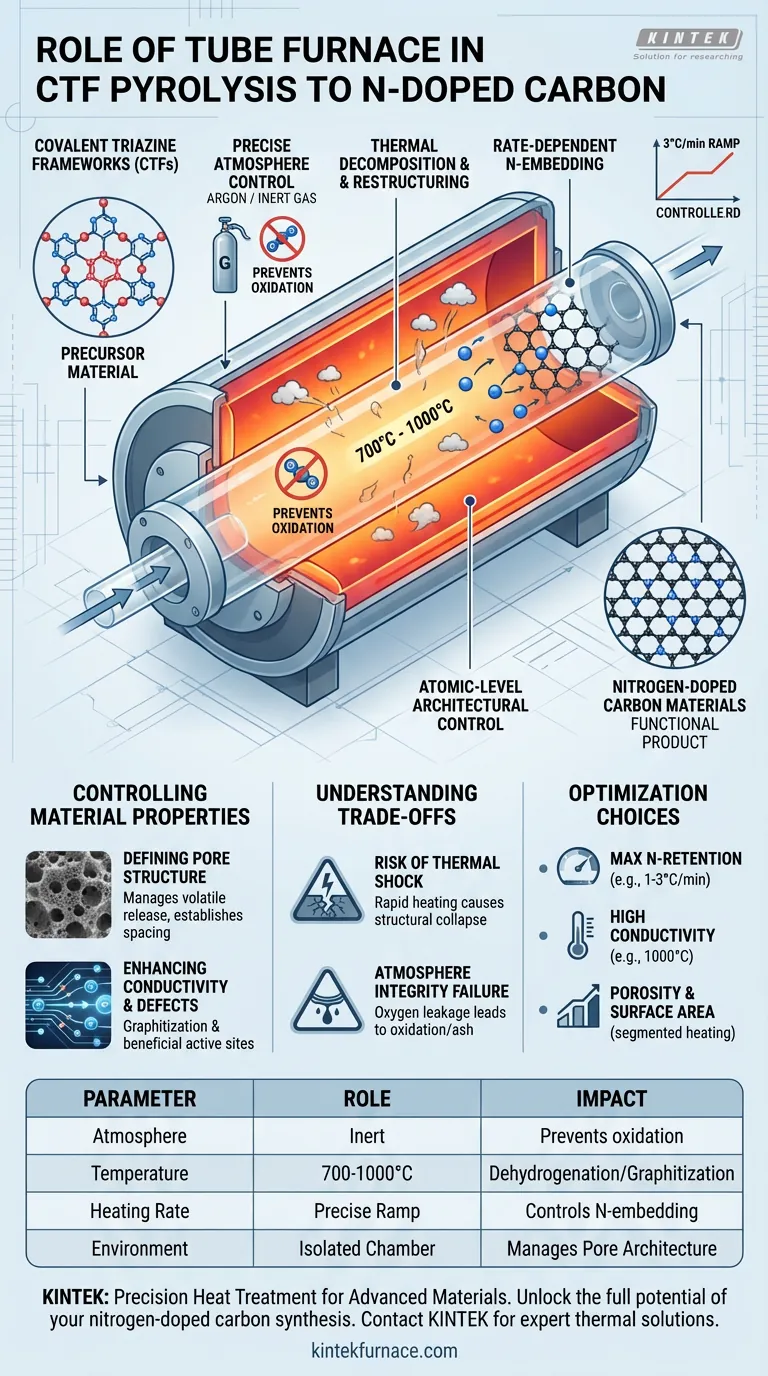
A tube furnace functions as the critical reaction chamber for converting covalent triazine frameworks (CTFs) into nitrogen-doped carbon materials. It provides the essential combination of a strictly inert atmosphere (such as argon) and precise thermal regulation between 700 °C and 1000 °C to restructure the material without destroying it.
Core Takeaway The tube furnace is not merely a heat source; it is a tool for atomic-level architectural control. Its ability to execute slow, precise heating rates under an oxygen-free atmosphere is the primary factor that allows nitrogen atoms to be embedded methodically into the carbon lattice, rather than being lost to oxidation or chaotic decomposition.

The Mechanism of Transformation
Precise Atmospheric Control
The most fundamental role of the tube furnace is to isolate the reaction environment. For the pyrolysis of CTFs, the furnace must maintain a strict inert atmosphere, typically using argon or nitrogen.
This prevents the material from reacting with oxygen. Without this exclusion of air, the high temperatures required for pyrolysis would simply burn the precursors, resulting in ash rather than the desired nitrogen-doped carbon.
Thermal Decomposition and Restructuring
The furnace acts as the driver for chemical metamorphosis. By sustaining temperatures ranging from 700 °C to 1000 °C, it provides the energy necessary to break specific chemical bonds within the CTF precursor.
This thermal energy triggers dehydrogenation and deoxygenation. As volatile components are removed, the furnace facilitates the recombination of the remaining elements into a stable, carbon-rich skeleton.
Rate-Dependent Nitrogen Embedding
The quality of the final material is dictated by the heating rate, which the tube furnace must control with high precision. A typical rate, such as 3 °C per minute, is often employed.
This controlled ramp rate is vital. It allows the nitrogen atoms to be "locked" into the carbon structure in an ordered fashion. If the heating is too aggressive, the nitrogen creates chaotic defects or escapes as gas, diminishing the doping effect.
Controlling Material Properties
Defining the Pore Structure
The furnace profile determines the physical architecture of the carbon. By managing the release of volatiles (gases escaping the material), the furnace establishes the preliminary pore structure.
Complex multi-stage heating profiles can be used to ensure this process happens gradually. This prevents the structural collapse of the material, resulting in a substrate with specific, useful interlayer spacing.
Enhancing Conductivity and Defects
High-temperature treatment within the furnace (often around 800 °C) transforms the polymer network into a graphitic or conductive carbon network.
Simultaneously, this harsh environment induces specific carbon vacancy defects. While "defects" sounds negative, in this context, they are often beneficial sites that increase the electrochemical activity of the material.
Understanding the Trade-offs
The Risk of Thermal Shock
While high temperatures are necessary, reaching them too quickly is detrimental. A furnace that lacks precise ramp control can cause thermal shock.
Rapid heating can lead to the structural collapse of the CTF before the carbon skeleton forms. This results in a material with low surface area and poor mechanical stability.
Atmosphere Integrity
The "tightness" of the tube furnace system is a common point of failure. Even trace amounts of oxygen leaking into the tube during the 700-1000 °C hold time can compromise the nitrogen doping.
If the atmosphere is not strictly inert, the carbon will oxidize. This leads to a loss of yield and a degradation of the electronic properties you are trying to engineer.
Making the Right Choice for Your Goal
To optimize the production of nitrogen-doped carbon from CTFs, tailor your furnace programming to your specific objectives:
- If your primary focus is maximum nitrogen retention: Prioritize a slower heating rate (e.g., 1–3 °C/min) to allow for the orderly integration of nitrogen atoms into the lattice.
- If your primary focus is high electrical conductivity: prioritize a higher final temperature (closer to 1000 °C) to maximize graphitization, accepting that total nitrogen content may slightly decrease.
- If your primary focus is porosity and surface area: Use a segmented heating program, holding at lower temperatures (e.g., 400 °C) to allow volatiles to escape gently before ramping to the final carbonization temperature.
The tube furnace is the instrument that bridges the gap between a raw chemical precursor and a functional, high-performance material.
Summary Table:
| Parameter | Role in CTF Pyrolysis | Impact on Final Material |
|---|---|---|
| Atmosphere | Strict Inert (Argon/Nitrogen) | Prevents oxidation; ensures carbon yield instead of ash |
| Temperature | 700 °C to 1000 °C | Facilitates dehydrogenation and graphitization |
| Heating Rate | Precise Ramp (e.g., 3 °C/min) | Controls nitrogen embedding and prevents structural collapse |
| Environment | Isolated Reaction Chamber | Manages volatile release to define pore architecture |
Precision Heat Treatment for Advanced Materials
Unlock the full potential of your nitrogen-doped carbon synthesis with KINTEK’s advanced thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to meet the rigorous demands of material science.
Our furnaces provide the precise ramp control and atmospheric integrity essential for managing covalent triazine framework transformations without compromising nitrogen retention or pore structure. Whether you require a standard setup or a fully customizable system for unique research needs, our team is ready to deliver the reliability your laboratory deserves.
Ready to elevate your material performance? Contact KINTEK today to consult with our experts on the perfect high-temp furnace for your application.
Visual Guide
References
- Xin Pan, Qianqian Zhu. Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol. DOI: 10.3390/nano14090744
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the disadvantages of resistance heating tube furnaces? Slow Heating, Uneven Temperatures, Low Efficiency
- What is the intended use of the 3-Zone tube furnace? Achieve Precise Thermal Control for Advanced Materials
- What critical role does a laboratory tube furnace play in pBN-CTF synthesis? Master Molecular Engineering
- Why is a quartz tube furnace used for two-stage LiFePO4 coating? Master Oxidation Control and Conductivity
- What role does a high-temperature tube furnace play in PEO to porous carbon conversion? Mastering Molecular Engineering
- What role does sodium hypophosphite (NaH2PO2) play in a tube furnace for NCMCP? Master Precise Phosphidation
- Why is a tube furnace required during the synthesis of phosphorus-doped nickel catalysts using high-purity nitrogen?
- What role does a dual-zone tube furnace play in TaAs2 single crystal growth? Master Precision Temperature Gradients



















