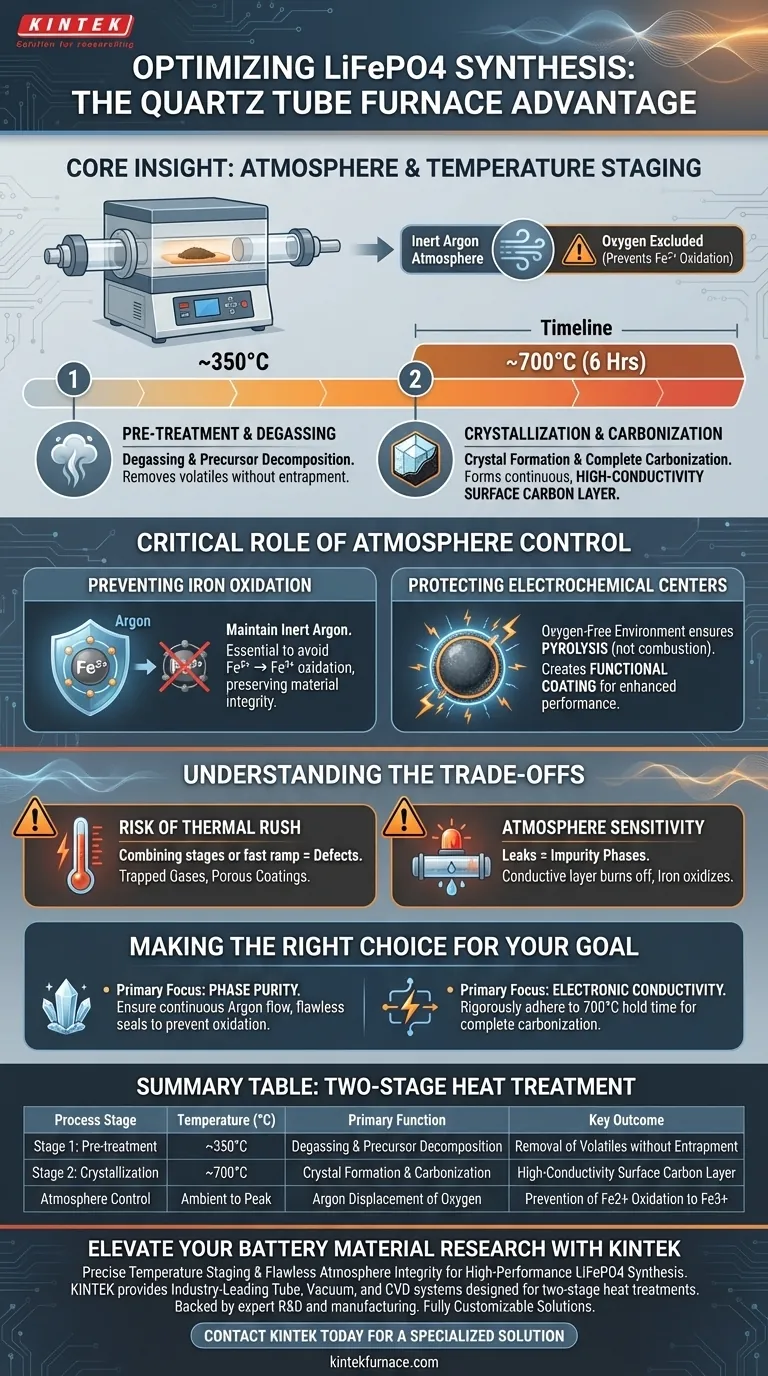
The utilization of a quartz tube furnace for LiFePO4 synthesis is driven by the critical need for a sealed, inert atmosphere and precise temperature staging to prevent oxidation and ensure conductivity. This equipment allows for a two-stage thermal process that protects the chemical integrity of the material while optimizing its surface properties.
Core Insight: The quartz tube furnace serves two distinct functions: it acts as a physical barrier to exclude oxygen (preserving the Fe2+ state) and provides a staged thermal ramp. This staging separates the volatile degassing of carbon precursors from the high-temperature crystallization of the final cathode material.

The Critical Role of Atmosphere Control
Preventing Iron Oxidation
The primary challenge in LiFePO4 synthesis is the instability of iron.
You must maintain an inert argon atmosphere throughout the heating process. The quartz tube furnace allows for the continuous displacement of oxygen, ensuring that divalent iron (Fe2+) does not oxidize into trivalent iron (Fe3+).
Protecting Electrochemical Centers
If oxygen penetrates the chamber, the electrochemical active centers of the lithium iron phosphate are compromised.
By maintaining an oxygen-free environment, the furnace ensures that the carbon sources undergo pyrolysis (thermal decomposition) rather than combustion. This is essential for creating a functional coating rather than burning off the carbon source.
Deconstructing the Two-Stage Heat Treatment
Stage 1: Pre-treatment and Degassing
The first stage of the process is conducted at approximately 350°C.
The objective here is the initial decomposition of carbon source precursors. This temperature facilitates degassing, allowing volatiles to escape before the material hardens.
Stage 2: Crystallization and Carbonization
The second stage involves heating the material to 700°C, typically for a duration of 6 hours.
This high-temperature phase drives the final formation of the LiFePO4 crystal structure. Simultaneously, it ensures the complete carbonization of mixed carbon sources, such as glucose and polystyrene.
The Resulting Surface Structure
The ultimate goal of this second stage is conductivity.
Proper execution results in a continuous and highly conductive surface carbon layer. This layer is vital for overcoming the naturally low electronic conductivity of phosphate-based cathode materials.
Understanding the Trade-offs
The Risk of Thermal Rush
Attempting to combine these stages or ramping temperature too quickly can lead to defects.
If the carbon source is not allowed to degas at 350°C, gases may become trapped during the 700°C crystallization phase, leading to porous or uneven coatings.
Atmosphere Sensitivity
The quartz tube system is highly effective but sensitive to leaks.
Even a minor breach in the argon seal can lead to the formation of impurity phases. If the environment is not strictly inert, the conductive carbon layer may burn off, and the iron will oxidize, rendering the material electrochemically inferior.
Making the Right Choice for Your Goal
To maximize the performance of your LiFePO4 material, align your process parameters with your specific quality targets:
- If your primary focus is Phase Purity: Ensure your argon flow is continuous and the tube seals are flawless to strictly prevent Fe2+ to Fe3+ oxidation.
- If your primary focus is Electronic Conductivity: rigorously adhere to the 700°C hold time to guarantee complete carbonization of the glucose or polystyrene precursors.
Success in LiFePO4 synthesis relies not just on the heat, but on the precise separation of degassing and crystallization within a protected environment.
Summary Table:
| Process Stage | Temperature (°C) | Primary Function | Key Outcome |
|---|---|---|---|
| Stage 1: Pre-treatment | ~350°C | Degassing & precursor decomposition | Removal of volatiles without entrapment |
| Stage 2: Crystallization | ~700°C | Crystal formation & carbonization | High-conductivity surface carbon layer |
| Atmosphere Control | Ambient to Peak | Argon displacement of Oxygen | Prevention of Fe2+ oxidation to Fe3+ |
Elevate Your Battery Material Research with KINTEK
Precise temperature staging and flawless atmosphere integrity are non-negotiable for high-performance LiFePO4 synthesis. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to meet the rigorous demands of two-stage heat treatments.
Backed by expert R&D and manufacturing, our lab high-temperature furnaces are fully customizable to your specific degassing and crystallization protocols. Don't compromise on phase purity or electronic conductivity.
Contact KINTEK Today for a Specialized Solution
Visual Guide
References
- Da Eun Kim, Yong Joon Park. Improving the Electrochemical Properties of LiFePO4 by Mixed-source-derived Carbon Layer. DOI: 10.33961/jecst.2025.00213
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is a fluidized bed vertical tube furnace? Achieve Superior Heating for Particulate Materials
- What are the controlled atmosphere capabilities of a tube furnace? Unlock Precise Gas Control for Your Lab
- What are the current market trends for 70mm tube furnaces? Discover Key Drivers in Automation and High-Tech Applications
- What is the function of a laboratory tube furnace in Ti-5Al-4W-2Fe alloy forging? Enhance Thermoplasticity & Purity
- What is the core function of a tube furnace in EN-LCNF synthesis? Unlock Precision Nanosheet Frameworks
- Why is calcination in a tube furnace necessary for pre-treating ZnS nanopowders? Achieve Optical Purity & Stability
- What is a drop tube furnace? Ideal for studying rapid combustion and ignition processes.
- What are the key benefits of using a tube furnace for material processing? Achieve Precise Heat Control for Superior Results



















