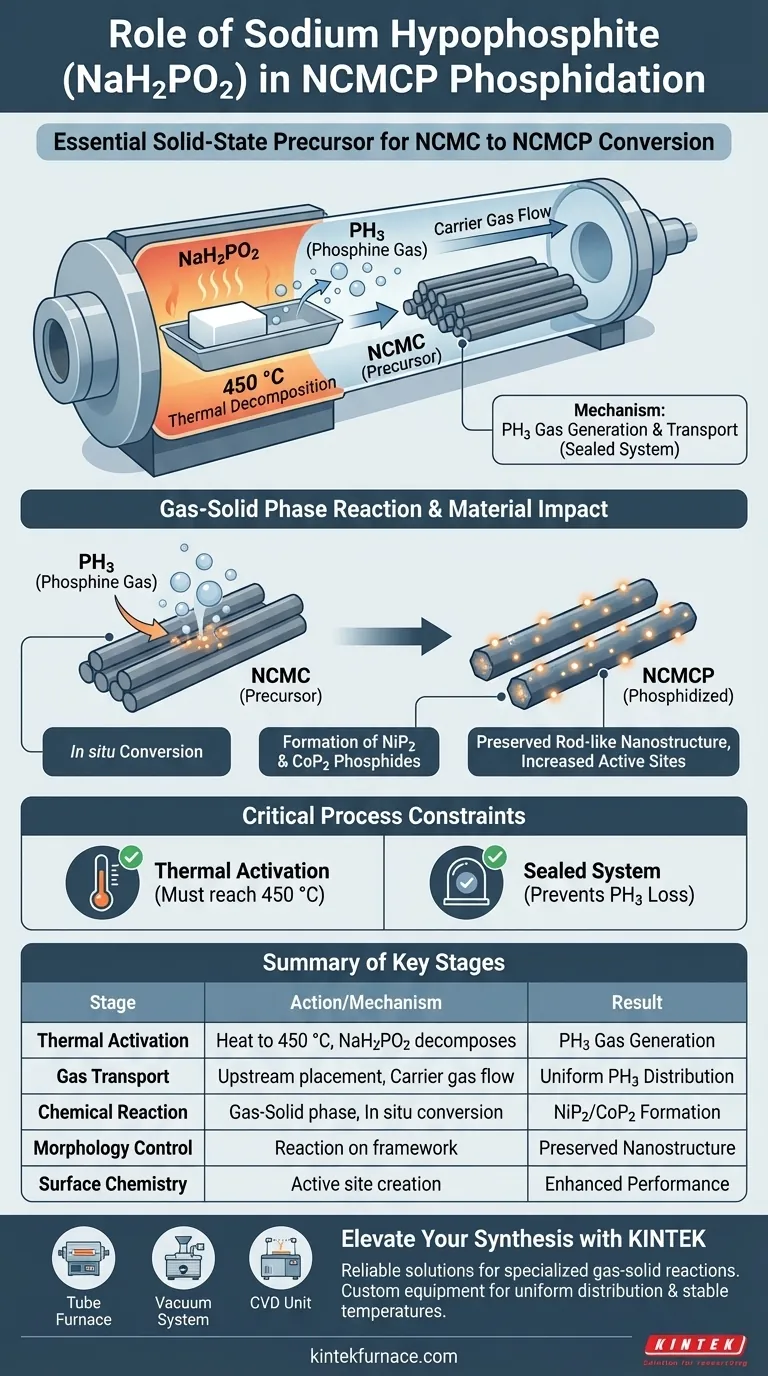
Sodium hypophosphite (NaH2PO2) acts as the essential solid-state precursor that drives the phosphidation of NCMC into NCMCP. When heated to 450 °C, it decomposes to release phosphine (PH3) gas, which serves as the reactive agent in a gas-solid phase transformation. This process chemically alters the material into transition metal phosphides while strictly preserving its physical architecture.
The core function of sodium hypophosphite is to provide a controlled, reactive phosphine atmosphere within a sealed system. This enables the in situ conversion of metal components into highly active phosphides (NiP2 and CoP2) without destroying the original rod-like nanostructure.

The Mechanism of Phosphidation
Thermal Decomposition
The process begins when the tube furnace reaches a specific activation temperature, typically 450 °C. At this threshold, the solid sodium hypophosphite undergoes thermal decomposition.
Generation of Reactive Gas
This decomposition releases phosphine (PH3) gas. Because the furnace provides a sealed flow reaction space, this gas is contained and directed rather than lost to the environment.
Strategic Upstream Placement
To ensure the reaction is effective, the sodium hypophosphite is typically placed at the upstream position of the boat within the furnace. This allows the carrier gas to transport the generated PH3 uniformly across the downstream NCMC material, ensuring deep penetration and consistent coverage.
Impact on Material Properties
Gas-Solid Phase Reaction
The PH3 gas interacts directly with the solid NCMC precursors. This gas-solid reaction is an in situ conversion process, meaning the transformation happens directly on the existing material framework.
Formation of Metal Phosphides
During this reaction, the metal components within the precursor are chemically transformed. Specifically, they convert into transition metal phosphides, such as NiP2 and CoP2.
Preservation of Morphology
Crucially, this chemical change does not alter the physical shape of the material. The original rod-like morphology is maintained, ensuring that the structural framework designed in earlier steps remains intact.
Increasing Active Sites
The conversion to phosphides significantly alters the surface chemistry of the material. This transformation creates a higher density of active sites, which is vital for the material's subsequent electrochemical performance.
Critical Process Constraints
Reliance on Thermal Activation
The reaction is entirely temperature-dependent. Without reaching the 450 °C threshold, the sodium hypophosphite will not decompose sufficiently to release the necessary phosphine gas, rendering the process ineffective.
Requirement for a Sealed System
The tube furnace must provide a sealed environment. Because the reactant is a gas (PH3), any breach in the seal would result in the loss of the reactive agent and inconsistent phosphidation of the sample.
Optimizing Your Synthesis Strategy
To ensure high-quality NCMCP preparation, consider the following operational priorities:
- If your primary focus is Compositional Purity: Ensure the furnace temperature is maintained at 450 °C to drive the complete decomposition of NaH2PO2 into reactive phosphine gas.
- If your primary focus is Uniformity: Place the sodium hypophosphite upstream from your samples to utilize the carrier gas for even distribution of phosphorus throughout the array.
By strictly controlling the thermal decomposition of sodium hypophosphite, you achieve a precise chemical upgrade of your material while protecting its physical geometry.
Summary Table:
| Stage | Action/Mechanism | Result for NCMCP |
|---|---|---|
| Thermal Activation | Heating to 450 °C | Decomposition of NaH2PO2 into PH3 gas |
| Gas Transport | Upstream placement | Uniform PH3 flow via carrier gas |
| Chemical Reaction | Gas-Solid phase transformation | Formation of NiP2 and CoP2 phosphides |
| Morphology Control | In situ conversion | Preservation of rod-like nanostructure |
| Surface Chemistry | Active site enrichment | Enhanced electrochemical performance |
Elevate Your Material Synthesis with KINTEK
Precise phosphidation requires rigorous thermal control and a perfectly sealed environment. KINTEK provides industry-leading Tube Furnaces, Vacuum Systems, and CVD units designed to handle specialized gas-solid reactions like NaH2PO2 decomposition with absolute reliability.
Our equipment is backed by expert R&D and is fully customizable to meet your unique lab requirements, ensuring uniform gas distribution and stable temperature thresholds for high-performance NCMCP preparation.
Ready to optimize your synthesis process? Contact KINTEK today for a custom solution!
Visual Guide
References
- Muhammad Ahsan Naseeb, Amir Waseem. Molybdenum carbide supported metal–organic framework-derived Ni, Co phosphosulphide heterostructures as efficient OER and HER catalysts. DOI: 10.1039/d5na00510h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- How can tube furnaces be enhanced with multiple heating zones and what benefits does this provide? Unlock Precise Temperature Control
- What is the specific function of a high-temperature tube furnace for MXene-NiCo2Se4? Master the Selenization Process
- What conditions does a tube vacuum furnace provide for zinc sulfide distillation? Optimize Your Zinc Ore Processing
- What is the purpose of introducing high-purity nitrogen into a tube furnace? Optimize Bone Pyrolysis & Biochar Yield
- How does the design of a dual-zone Tube Furnace facilitate precise metal phosphide conversion? Optimize Heterojunctions
- Why must the atmosphere be strictly controlled to 10% O2/Ar in a tube furnace for BiFeO3? Achieve Pure Phase Results
- What is the necessity of annealing treatment for CuCo2O4@rGO? Optimize High-Crystallinity Synthesis in Tube Furnaces
- How does a tube furnace facilitate the activation of xylan-derived carbon spheres? Precision Surface Engineering



















