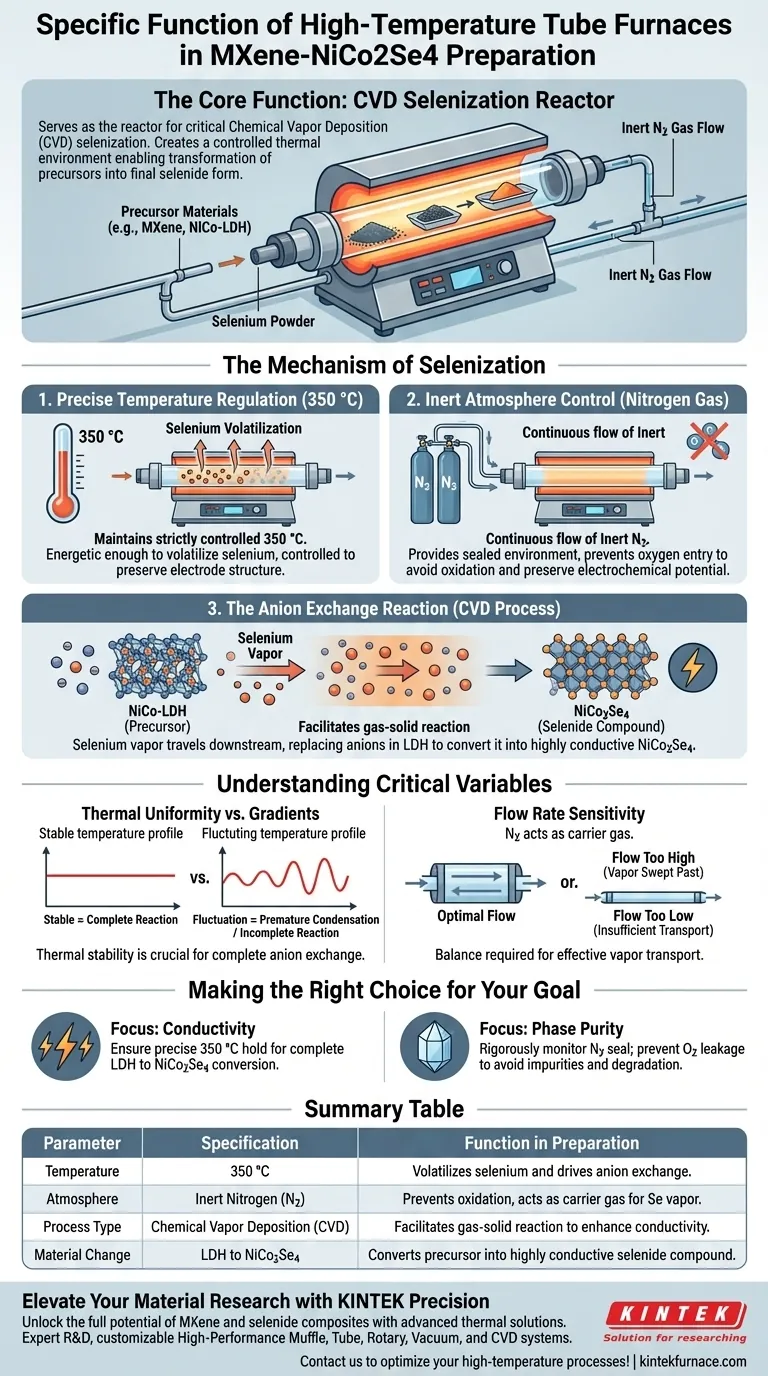
The high-temperature tube furnace serves as the reactor for the critical chemical vapor deposition (CVD) selenization process. It creates a controlled thermal environment that enables the transformation of precursor materials into their final selenide form. By maintaining a specific temperature under an inert atmosphere, the furnace facilitates the chemical reaction required to enhance the material's electrical properties.
The furnace drives an anion exchange reaction at 350 °C under nitrogen, converting NiCo-LDH into conductive NiCo2Se4. Its primary function is to facilitate the volatilization of selenium and ensure its uniform reaction with the electrode precursor.

The Mechanism of Selenization
Precise Temperature Regulation
The tube furnace must maintain a strictly controlled operating temperature of 350 °C.
At this specific thermal point, selenium powder placed within the tube volatilizes into vapor. This temperature is energetic enough to drive the reaction but controlled enough to preserve the structural integrity of the underlying electrode material.
Inert Atmosphere Control
The process operates under a continuous flow of inert nitrogen gas.
The tube furnace provides a sealed environment that prevents oxygen from entering the system. This is vital to ensure that the volatilized selenium reacts with the precursor rather than oxidizing, which would ruin the material's electrochemical potential.
The Anion Exchange Reaction
The core function of the furnace is to facilitate a gas-solid chemical reaction.
The selenium vapor travels downstream to the NiCo-LDH (Layered Double Hydroxide) precursor. A chemical vapor deposition (CVD) process occurs where selenium anions replace the existing anions in the LDH structure. This exchange converts the precursor into NiCo2Se4, a selenide compound with significantly higher electrical conductivity.
Understanding the Critical Variables
Thermal Uniformity vs. Gradients
While the target temperature is 350 °C, the success of the CVD process depends on thermal stability.
A common pitfall in tube furnace operations is an uneven thermal zone. If the temperature fluctuates or drops across the tube length, the selenium may condense prematurely or fail to react completely with the NiCo-LDH.
Flow Rate Sensitivity
The inert nitrogen atmosphere does more than just protect the sample; it acts as a carrier gas.
If the gas flow is too high, selenium vapor may be swept past the precursor too quickly for the anion exchange to occur. If the flow is too low, vapor transport may be insufficient. The furnace setup must balance temperature with precise gas flow dynamics.
Making the Right Choice for Your Goal
Application Recommendations
Depending on your specific research or production focus, prioritize the following parameters:
- If your primary focus is conductivity: Ensure the furnace holds 350 °C precisely to guarantee the complete conversion of chemically resistive LDH into highly conductive NiCo2Se4.
- If your primary focus is phase purity: Monitor the nitrogen seal rigorously, as any oxygen leakage during the high-temperature phase will introduce impurities and degrade the selenide structure.
The tube furnace is not just a heater; it is the active reaction chamber that dictates the chemical identity and performance of your final composite material.
Summary Table:
| Parameter | Specification | Function in MXene-NiCo2Se4 Preparation |
|---|---|---|
| Temperature | 350 °C | Volatilizes selenium and drives anion exchange reaction |
| Atmosphere | Inert Nitrogen ($N_2$) | Prevents oxidation and acts as a carrier gas for Se vapor |
| Process Type | Chemical Vapor Deposition (CVD) | Facilitates gas-solid reaction to enhance conductivity |
| Material Change | LDH to $NiCo_2Se_4$ | Converts precursor into highly conductive selenide compound |
Elevate Your Material Research with KINTEK Precision
Unlock the full potential of your MXene and selenide composites with KINTEK’s advanced thermal solutions. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific research or production needs.
Whether you require precise thermal uniformity for anion exchange or rigorous atmosphere control for sensitive electrode precursors, KINTEK provides the reliability and technical expertise your lab demands. Contact us today to optimize your high-temperature processes!
Visual Guide
References
- Hui Li, Min Jae Ko. Selenized Binary Transition Metals‐MXene Composite for High‐Performance Asymmetric Hybrid Capacitors. DOI: 10.1002/smll.202504350
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does the direct annealing process in a tube furnace affect iridium-based inverse opals? Expert Insights
- What is a tubular furnace and what are its primary uses? Essential for High-Temperature Precision and Uniformity
- What is a drop tube furnace and what is its primary purpose? Master Rapid Thermal Processing for Particle Studies
- What role does a high-vacuum tube furnace (CVD) play in HEA@CNT synthesis? Master Nanocomposite In-Situ Growth
- What is sintering, and how is it performed in horizontal furnaces? Unlock Precision in Powder Processing
- What is the role of high-temperature tube furnaces in the post-processing of graphite oxide nanostructures?
- What advantages does a fluidized bed vertical tube furnace offer in terms of operation? Unlock Superior Heat Transfer and Uniformity
- What core physical conditions does a tube furnace provide in the two-step synthesis of WS2? Master Film Growth



















