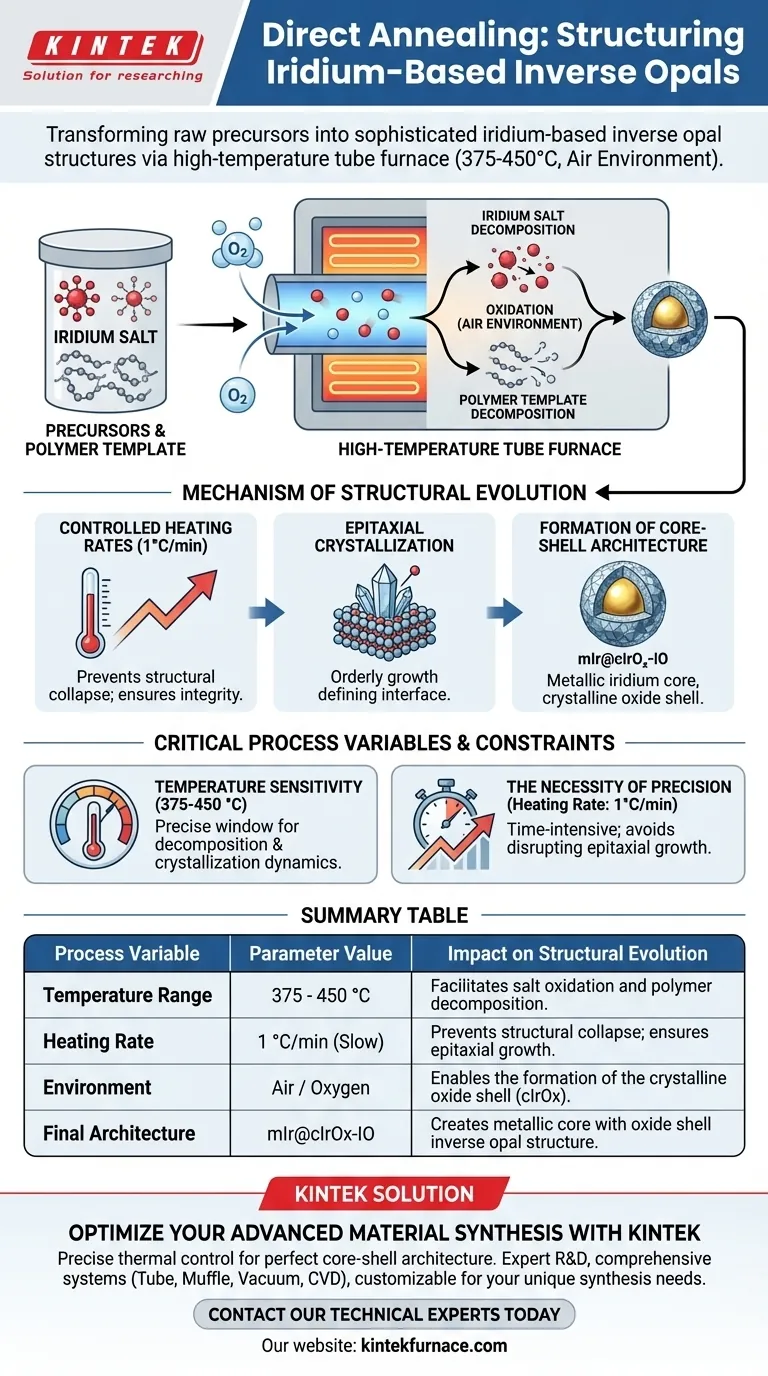
The direct annealing process in a high-temperature tube furnace is the primary driver for transforming raw precursors into sophisticated iridium-based inverse opal structures. By operating between 375 and 450 degrees Celsius in an air environment, the furnace facilitates the simultaneous decomposition of the polymer template and the oxidation of iridium salts.
The core mechanism relies on precise thermal control to induce epitaxial crystallization, creating a unique core-shell architecture composed of a metallic iridium center and an oxide shell (mIr@cIrOx-IO).

The Dual Role of Thermal Treatment
To understand the structural evolution, you must first recognize that the tube furnace performs two distinct chemical functions at the same time.
Simultaneous Decomposition
The thermal energy provided by the furnace initiates the breakdown of the iridium salt precursors.
Concurrently, the heat induces the thermal decomposition of the polymer template, which is responsible for the inverse opal shape.
Environmental Influence
Because this process takes place in an air environment, oxygen is readily available during the heating cycle.
This ensures that as the precursor decomposes, the iridium species are immediately subjected to oxidation.
Mechanism of Structural Evolution
The way the structure forms is not random; it is dictated by the specific application of heat over time.
Controlled Heating Rates
The structural integrity of the final material depends heavily on a slow, controlled heating rate, such as 1 degree Celsius per minute.
This gradual increase allows the materials to evolve without the structural collapse that might occur with rapid thermal shock.
Epitaxial Crystallization
Under these controlled conditions, the furnace induces epitaxial crystallization of the iridium species.
This orderly crystal growth is critical for defining the interface between the different phases of the material.
Formation of the Core-Shell Architecture
The ultimate result of this process is the formation of a mIr@cIrOx-IO structure.
This denotes a specific configuration where a metallic iridium core is encapsulated by a crystalline oxide shell, evolved directly from the single annealing step.
Critical Process Variables and Constraints
While effective, this process relies on strict adherence to specific parameters to avoid failure.
Temperature Sensitivity
The process requires a precise window between 375 and 450 degrees Celsius.
Operating outside this range may fail to achieve the necessary decomposition or could alter the crystallization dynamics unfavorably.
The Necessity of Precision
The reliance on a specific heating rate implies that this is a time-intensive process.
Rushing the ramp rate risks disrupting the epitaxial growth, which would prevent the formation of the distinct core-shell morphology.
Optimizing the Annealing Protocol
To replicate this structural evolution successfully, you must align your furnace parameters with the material's thermal requirements.
- If your primary focus is Structural Definition: Adhere strictly to the slow heating rate (e.g., 1°C/min) to ensure the polymer template decomposes without collapsing the inorganic framework.
- If your primary focus is Phase Composition: Maintain the temperature between 375 and 450°C to guarantee the correct balance of metallic core retention and oxide shell formation.
Precise thermal management in the tube furnace is the defining factor in successfully synthesizing this dual-phase iridium architecture.
Summary Table:
| Process Variable | Parameter Value | Impact on Structural Evolution |
|---|---|---|
| Temperature Range | 375 - 450 °C | Facilitates salt oxidation and polymer decomposition. |
| Heating Rate | 1 °C/min (Slow) | Prevents structural collapse; ensures epitaxial growth. |
| Environment | Air / Oxygen | Enables the formation of the crystalline oxide shell (cIrOx). |
| Final Architecture | mIr@cIrOx-IO | Creates metallic core with oxide shell inverse opal structure. |
Optimize Your Advanced Material Synthesis with KINTEK
Precise thermal control is the difference between a collapsed framework and a perfect core-shell architecture. At KINTEK, we understand the rigorous demands of epitaxial crystallization and structural evolution in iridium-based materials.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Tube, Muffle, Vacuum, and CVD systems. Our high-temperature furnaces are fully customizable to meet your specific ramp rates and atmospheric requirements, ensuring your research yields consistent, high-performance results.
Ready to elevate your lab's heating precision? Contact our technical experts today to discuss your unique synthesis needs.
Visual Guide
References
- Sebastian Möhle, Peter Strasser. Iridium Oxide Inverse Opal Anodes with Tailored Porosity for Efficient PEM Electrolysis. DOI: 10.1002/adfm.202501261
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the key advantages of using fluidized bed technology in vertical tube furnaces? Boost Efficiency and Uniformity
- What temperature should the furnace be at when loading or unloading samples? Stay Safe and Prevent Damage
- What are the handling and placement precautions for a vacuum tube furnace? Ensure Safe and Efficient Operation
- What types of heating elements are commonly used in drop tube furnaces? Find the Right Element for Your Temperature Needs
- What common processes are enabled by tube furnaces? Unlock Precise Thermal Processing for Your Lab
- What are the key features of three-zone tube furnaces? Unlock Precision for Advanced Materials Processing
- What is the role of a vacuum tube furnace during the final thermal treatment stage of Fe3O4@CSAC catalysts?
- What is the difference between an alumina tube furnace and a quartz tube furnace? Choose the Right Tube Furnace for Your Lab



















