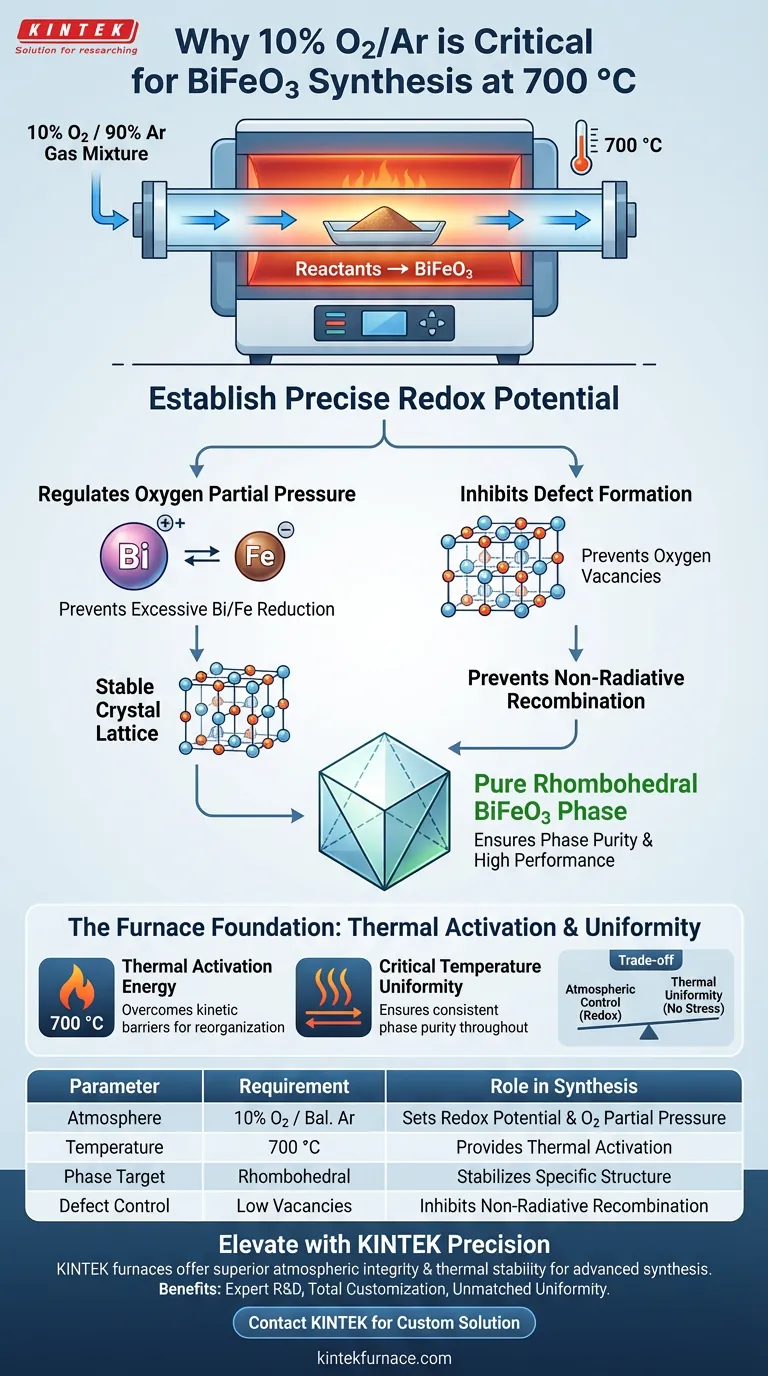
Strict atmospheric control using 10% O2/Ar is essential because it establishes the precise redox potential required to stabilize the BiFeO3 crystal structure during synthesis at 700 °C. This specific gas mixture regulates the oxygen partial pressure to prevent the excessive reduction of bismuth and iron ions, which is critical for forming the pure rhombohedral phase.
By maintaining a specific oxygen partial pressure, the furnace atmosphere inhibits the formation of oxygen vacancies and unwanted ion reduction. This ensures the resulting BiFeO3 is phase-pure and free from defects that lead to performance-degrading non-radiative recombination.

The Role of Redox Potential in Crystal Growth
To achieve high-quality BiFeO3, you cannot simply rely on temperature; you must actively manage the chemical environment within the tube furnace.
Regulating Oxygen Partial Pressure
The 10% O2/Ar mixture creates a specific redox potential within the furnace chamber. This environment is carefully tuned to balance the oxidation state of the reactants.
Without this precise partial pressure, the chemical equilibrium shifts, potentially destabilizing the crystal lattice as it forms.
Inhibiting Excessive Ion Reduction
In high-bismuth systems, the stability of metal ions is a primary concern. The controlled atmosphere specifically inhibits the excessive reduction of bismuth or iron ions.
If these ions are reduced beyond their target valency, the material fails to form the intended compound, leading to impurities or structural collapse.
Ensuring Phase Purity and Performance
The ultimate goal of using this specific atmosphere is to dictate the physical and electronic properties of the final material.
Stabilizing the Rhombohedral Phase
The synthesis process targets a specific crystal arrangement known as the pure rhombohedral BiFeO3 phase.
The 10% O2/Ar atmosphere provides the thermodynamic conditions necessary for this specific phase to nucleate and grow stably at 700 °C.
Preventing Oxygen Vacancies
Defects in the crystal lattice, specifically oxygen vacancies, are detrimental to the material's performance.
By maintaining sufficient oxygen partial pressure, the process fills the lattice correctly, preventing vacancies that would otherwise act as centers for non-radiative recombination.
The Function of the Tube Furnace Environment
While the gas mixture controls the chemistry, the high-temperature tube furnace provides the physical foundation for synthesis.
Thermal Activation Energy
The furnace supplies the necessary thermal activation energy to drive the reaction between reactants.
At 700 °C, the precursors have enough energy to overcome kinetic barriers and reorganize into the complex BiFeO3 structure.
Critical Temperature Uniformity
A high degree of temperature uniformity within the furnace zones is required to ensure the reaction proceeds identically throughout the sample.
Uniform heat distribution prevents local variations in phase purity, ensuring the entire sample achieves the desired complete crystal structure.
Understanding the Trade-offs
Precise atmospheric control is a delicate balancing act that requires vigilance.
The Risks of Improper Partial Pressure
If the oxygen concentration deviates from the 10% standard, you risk compromising the redox potential.
Too little oxygen leads to the reduction defects mentioned earlier, while incorrect ratios may fail to stabilize the rhombohedral phase entirely.
Thermal Uniformity vs. Stress
While high heat is necessary for activation, thermal stress is a potential byproduct if the heating environment is unstable.
Although the primary goal is phase purity, the furnace must maintain a stable thermal profile to prevent morphological non-uniformity in the final product.
Making the Right Choice for Your Goal
The parameters you select in your tube furnace largely depend on the specific material properties you need to optimize.
- If your primary focus is Structural Integrity: Adhere strictly to the 10% O2/Ar ratio to inhibit ion reduction and ensure the formation of the stable rhombohedral phase.
- If your primary focus is Electronic Efficiency: Prioritize precise oxygen partial pressure control to eliminate oxygen vacancies and prevent non-radiative recombination.
Mastering the balance between thermal activation and atmospheric redox potential is the key to synthesizing defect-free BiFeO3.
Summary Table:
| Parameter | Requirement | Role in BiFeO3 Synthesis |
|---|---|---|
| Atmosphere | 10% O2 / Balance Ar | Establishes precise redox potential and oxygen partial pressure. |
| Temperature | 700 °C | Provides thermal activation energy for phase nucleation. |
| Phase Target | Rhombohedral | Controlled atmosphere stabilizes this specific crystal structure. |
| Ion Stability | High | Prevents excessive reduction of bismuth and iron ions. |
| Defect Control | Low Vacancies | Inhibits oxygen vacancies to prevent non-radiative recombination. |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect 10% O2/Ar environment requires more than just gas; it demands a furnace with superior atmospheric integrity and thermal stability. KINTEK provides industry-leading Tube, Muffle, Rotary, and Vacuum systems designed for the rigorous demands of advanced material research.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our systems are engineered to maintain strict redox potentials and oxygen partial pressures.
- Total Customization: We tailor high-temperature solutions to meet your specific rhombohedral phase requirements.
- Unmatched Uniformity: Ensure consistent phase purity across every sample with our advanced heating zone technology.
Ready to eliminate defects and ensure phase-pure results? Contact KINTEK today for a custom furnace solution!
Visual Guide
References
- Yuanjun Song, Tong Zhang. A Simple One-Pot Method for the Synthesis of BiFeO3/Bi25FeO40 Heterojunction for High-Performance Photocatalytic Degradation Applications. DOI: 10.3390/ijms26010196
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What certifications are associated with three-zone split tube furnaces? Key Marks for Quality and Safety
- What is the purpose of introducing nitrogen flow into a tube furnace? Optimize Your Activated Carbon Calcination
- How does a tube furnace contribute to the CVD of Si-SiO2 composites? Achieve Precise Nanostructure Control
- How does a tube furnace achieve energy efficiency? Optimize Heat Retention and Control
- What are the key design features of a split tube furnace? Unlock Superior Access for Complex Experiments
- What advantages do three-zone furnaces offer? Achieve Superior Temperature Control and Efficiency
- What is the purpose of the gas circulation system in a tube furnace? Control Chemical Atmospheres for Precise High-Temperature Processing
- Why is a high-precision dual-zone furnace required for 1T-TaS2 crystals? Achieve Perfect CVT Phase Integrity



















