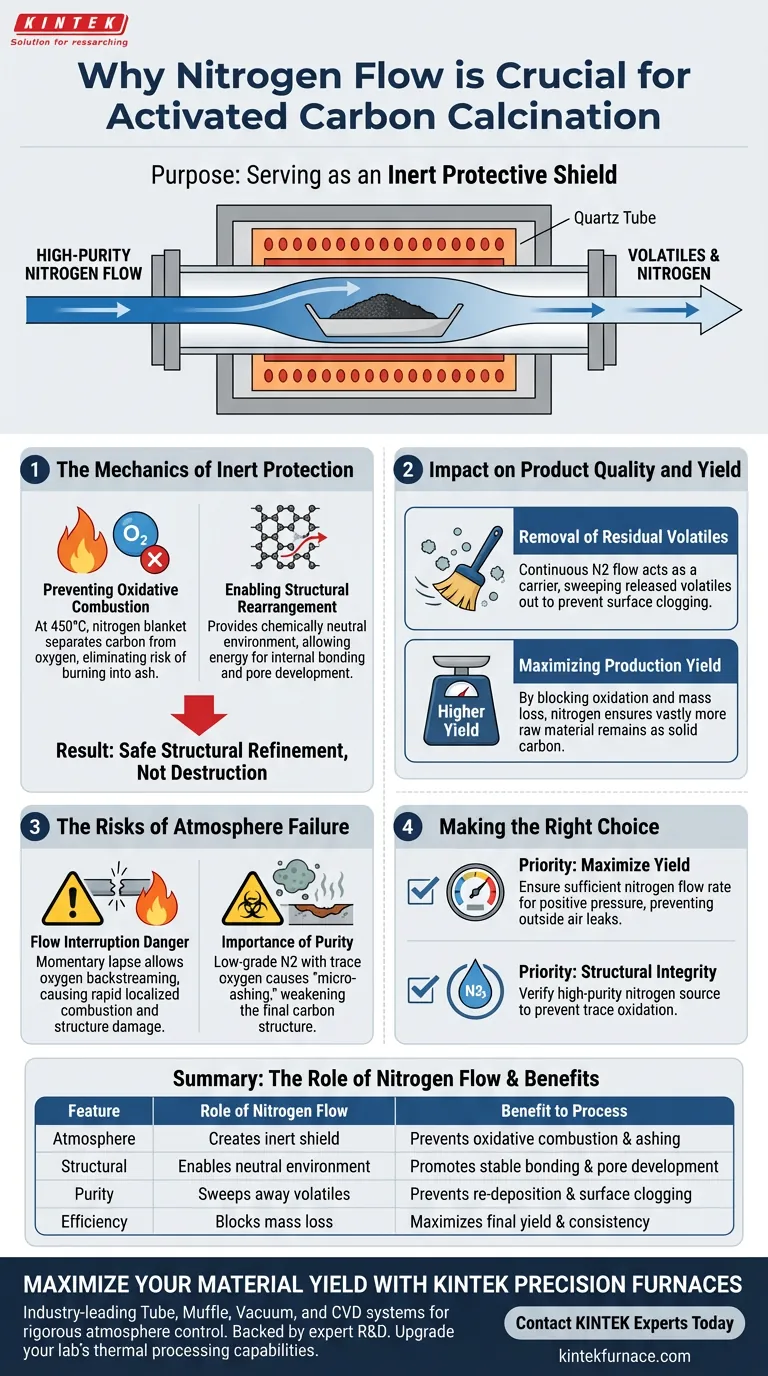
The primary purpose of nitrogen flow is to serve as an inert protective shield. By displacing oxygen within the tube furnace, specifically during high-temperature calcination at 450°C, nitrogen prevents the carbon material from reacting with air. This stops the raw material from burning into ash (oxidative combustion) while allowing the necessary internal chemical changes to occur safely.
By maintaining an oxygen-free environment, you ensure the carbon material undergoes structural refinement rather than destruction, significantly preserving the material's mass and maximizing the final yield.

The Mechanics of Inert Protection
Preventing Oxidative Combustion
At calcination temperatures of 450°C, carbon is highly reactive with oxygen. Without a protective atmosphere, the carbon material would ignite and burn away.
High-purity nitrogen creates a blanket that physically separates the carbon from oxygen. This effectively eliminates the risk of the material turning into ash during the heating process.
Enabling Structural Rearrangement
The goal of calcination is to refine the internal structure of the material. The carbon framework needs to reorganize itself to become stable and porous.
Nitrogen provides the chemically neutral environment required for this process. It ensures that the energy applied to the system is used for internal bonding and restructuring, rather than fueling a combustion reaction.
Impact on Product Quality and Yield
Removal of Residual Volatiles
During the heating process, volatile components trapped within the raw material must be expelled. If these remain, they clog the pores of the final activated carbon.
The continuous flow of nitrogen acts as a carrier mechanism. It actively sweeps these released volatiles out of the furnace zone, preventing them from re-depositing on the carbon surface.
Maximizing Production Yield
The presence of oxygen leads to mass loss through burning. Every gram of carbon that combusts is a gram of lost product.
By blocking oxidation, nitrogen ensures that the vast majority of the raw material remains as solid carbon. This directly correlates to a higher final yield of activated carbon relative to the starting material.
The Risks of Atmosphere Failure
The Danger of Flow Interruption
The protection provided by nitrogen relies on a continuous flow. Even a momentary lapse in the nitrogen stream can allow oxygen to backstream into the furnace.
At 450°C, this reintroduction of oxygen can cause rapid, localized combustion. This damages the pore structure of the carbon and reduces the overall batch yield immediately.
The Importance of Purity
The reference specifies high-purity nitrogen for a reason. Nitrogen supplies contaminated with trace amounts of oxygen or moisture can compromise the process.
Using low-grade nitrogen can lead to "micro-ashing," where the surface of the carbon degrades slightly, weakening the final structure even if total combustion is avoided.
Making the Right Choice for Your Goal
To ensure optimal calcination results, consider these priorities when setting up your furnace atmosphere:
- If your primary focus is maximizing yield: Ensure the nitrogen flow rate is sufficient to maintain positive pressure, preventing any outside air from leaking into the heating zone.
- If your primary focus is structural integrity: Verify the purity of your nitrogen source to prevent trace oxidation from interfering with the carbon framework rearrangement.
Control the atmosphere, and you control the quality of the carbon.
Summary Table:
| Feature | Role of Nitrogen Flow | Benefit to Process |
|---|---|---|
| Atmosphere | Creates an inert protective shield | Prevents oxidative combustion and ashing |
| Structural | Enables neutral environment | Promotes stable bonding and pore development |
| Purity | Sweeps away released volatiles | Prevents re-deposition and surface clogging |
| Efficiency | Blocks mass loss from burning | Maximizes final yield and product consistency |
Maximize Your Material Yield with KINTEK Precision Furnaces
Don't let oxidative combustion compromise your research or production results. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed for rigorous atmosphere control. Backed by expert R&D and manufacturing, our high-temp furnaces are fully customizable to handle high-purity nitrogen integration, ensuring your activated carbon and advanced materials undergo perfect structural refinement.
Ready to upgrade your lab's thermal processing capabilities?
Visual Guide
References
- Jolantje Latupeirissa, Muliana Muliana. CHARACTERISATION OF ACTIVATED CARBON FROM WHITE SNAPPER SCALES (Lates calcarife) WASTE. DOI: 10.30872/jkm.v21i2.1292
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- How does a tube furnace facilitate the growth of controlled oxide layers on X70 carbon steel? Engineering Precision
- Why is a high-vacuum sealed quartz tube used in CVT? Ensuring High-Purity Fe4GeTe2 Single Crystal Growth
- What role does a tubular furnace play in the synthesis of Si:B nanowires? Driving Thermal Evaporation and Growth
- What is the process for using a vacuum tube experimental furnace? Master Precise Control for Your Lab
- What is the function of a tube furnace in catalyst annealing? Unlock L10 Ordered Structures for Peak Performance
- What role does a high-temperature tube furnace play in the pore expansion of porous graphene? Expert Material Engineering
- How is the structure of a multi station vacuum tube furnace divided? Optimize Your Lab's Thermal Processing
- How does a high-precision tube furnace contribute to the reduction process of Cu/ZIF-8 catalysts?



















