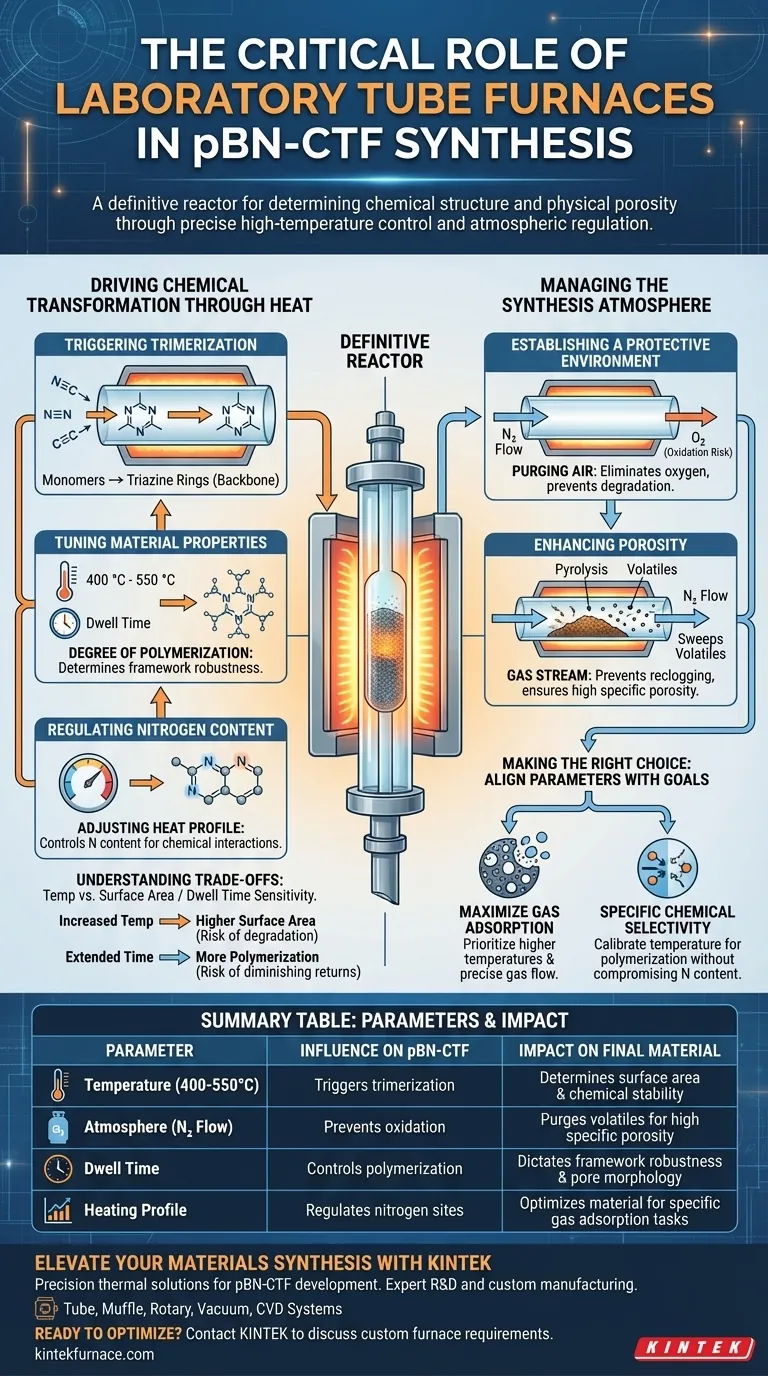
A laboratory tube furnace acts as the definitive reactor for the synthesis of pBN-CTF, serving as the critical vessel where chemical structure and physical porosity are determined. It provides the precise high-temperature environment necessary to trigger the cyclotrimerization of nitrile monomers into the 1,3,5-triazine rings that form the material's backbone.
By tightly regulating both thermal energy and atmospheric conditions, the tube furnace allows researchers to dictate the final polymerization degree, specific surface area, and nitrogen content. This control is the key to optimizing the material for specific gas adsorption applications.

Driving Chemical Transformation Through Heat
Triggering Trimerization
The primary function of the furnace is to supply the activation energy required for chemical synthesis. Specifically, it facilitates the trimerization of nitrile monomers.
This thermal process converts these monomers into stable 1,3,5-triazine rings. These rings are the fundamental structural units of pBN-CTF.
Tuning Material Properties
The furnace allows for the precise manipulation of heating temperatures—typically between 400 °C and 550 °C—and dwell times.
These variables directly influence the degree of polymerization. A higher degree of polymerization often results in a more robust framework.
Regulating Nitrogen Content
Temperature settings also dictate the elemental composition of the final product.
By adjusting the heat profile, you can control the nitrogen content within the matrix. This is vital, as nitrogen sites often play a key role in the material's chemical interactions and adsorption capabilities.
Managing the Synthesis Atmosphere
Establishing a Protective Environment
Beyond heat, the furnace's gas path control system is responsible for maintaining a stable atmosphere.
It delivers a constant flow of nitrogen to purge air from the chamber. This eliminates oxygen, preventing unwanted oxidation that could degrade the material during synthesis.
Enhancing Porosity
The continuous gas flow plays a mechanical role in determining surface area.
As the material undergoes pyrolysis, volatile components are generated. The gas stream actively sweeps these volatiles away, preventing them from reclogging the developing structure and ensuring high specific porosity.
Understanding the Trade-offs
Temperature vs. Surface Area
Increasing the furnace temperature generally enhances the specific surface area of the pBN-CTF.
However, aggressive heating must be balanced against chemical stability. Extremely high temperatures could potentially degrade the desired nitrogen functional groups.
Dwell Time Sensitivity
Extending the dwell time can lead to a more complete reaction and higher polymerization.
Conversely, excessive dwell times may yield diminishing returns or alter pore morphology in unintended ways. Precision in timing is just as critical as temperature selection.
Making the Right Choice for Your Synthesis Goal
To achieve the best results with pBN-CTF synthesis, align your furnace parameters with your specific material requirements:
- If your primary focus is maximizing gas adsorption: Prioritize higher temperatures (e.g., 550 °C) and precise gas flow to clear volatiles and maximize specific surface area.
- If your primary focus is specific chemical selectivity: Carefully calibrate the temperature to ensure a high degree of polymerization without compromising the material's nitrogen content.
Mastering the tube furnace parameters transforms the synthesis from a rough heating process into a precise engineering of molecular architecture.
Summary Table:
| Parameter | Influence on pBN-CTF | Impact on Final Material |
|---|---|---|
| Temperature (400-550°C) | Triggers trimerization | Determines surface area and chemical stability |
| Atmosphere (N₂ Flow) | Prevents oxidation | Purges volatiles to ensure high specific porosity |
| Dwell Time | Controls polymerization | Dictates framework robustness and pore morphology |
| Heating Profile | Regulates nitrogen sites | Optimizes material for specific gas adsorption tasks |
Elevate Your Materials Synthesis with KINTEK
Precision is the difference between a rough reaction and a high-performance material. KINTEK provides industry-leading thermal solutions designed for the rigorous demands of pBN-CTF development. Backed by expert R&D and manufacturing, we offer high-precision Tube, Muffle, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory needs.
Ready to optimize your polymerization degree and surface area? Contact KINTEK today to discuss your custom furnace requirements and leverage our expertise in high-temperature engineering.
Visual Guide
References
- Hanibal Othman, Christoph Janiak. Synthesis and Characterization of Covalent Triazine Frameworks Based on 4,4′-(Phenazine-5,10-diyl)dibenzonitrile and Its Application in CO2/CH4 Separation. DOI: 10.3390/molecules30153110
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What role do tube plugs and thermal fixtures play in vertical tube furnaces? Enhance Temperature Control and Versatility
- What is the primary function of introducing high-purity argon into the tube furnace? Expert Pyrolysis Solutions
- How does the temperature controller function in a 70mm tube furnace? Achieve Precise Thermal Control for Your Lab
- What is the purpose of using a high-temperature tube furnace with an argon atmosphere during carbonization?
- What are the technical advantages of using an oscillating tube furnace for tellurium dioxide recovery?
- What is a high temperature tube furnace? Achieve Precise Heat and Atmosphere Control
- How does a tube furnace facilitate the structural stabilization of lignin? Mastering Lignin-to-Carbon Transformation
- How is a Tube Furnace utilized to transform oxidized catalyst precursors into sulfided K-MoS2/Al2O3? Precise Activation



















