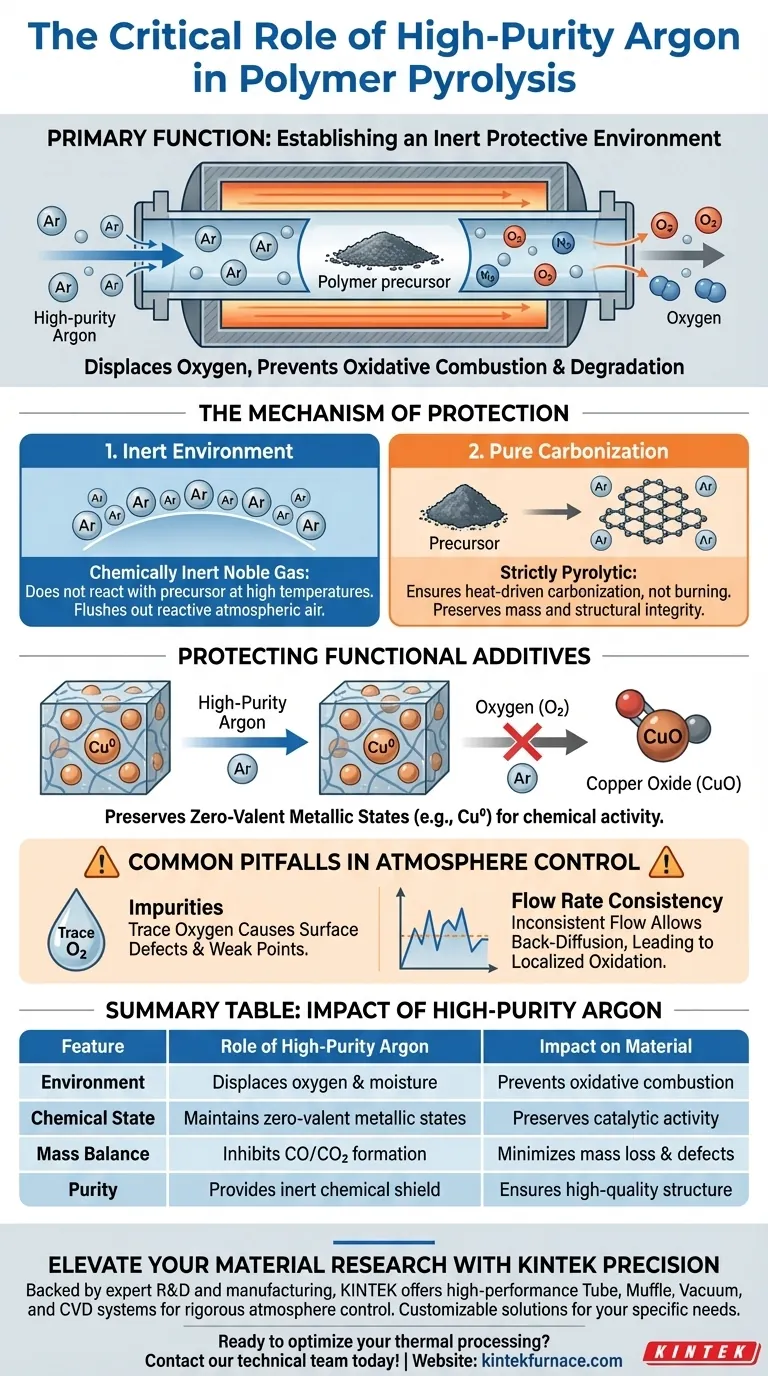
The primary function of introducing high-purity argon is to establish an inert protective environment within the tube furnace. By completely displacing oxygen, argon prevents the polymer precursor and the resulting pyrolytic carbon from undergoing oxidative combustion or degradation during the high-temperature heating process.
By excluding reactive gases, argon acts as a chemical shield that forces the precursor to undergo carbonization rather than combustion. This preserves the material's mass, ensures structural integrity, and maintains the chemical purity required for high-performance applications.

The Mechanism of Atmospheric Protection
Establishing an Inert Environment
Argon is a noble gas, meaning it is chemically inert and does not react with the polymer precursor even at the extreme temperatures required for pyrolysis.
By flowing high-purity argon through the furnace, you physically flush out atmospheric air. This removes oxygen and nitrogen, which are reactive elements that would otherwise interfere with the chemical transformation.
Preventing Oxidative Mass Loss
The most critical role of this inert atmosphere is stopping oxidative mass loss.
If oxygen were present, the carbon atoms generated during pyrolysis would react with it to form carbon dioxide or carbon monoxide gases. This would essentially burn the material away, leading to a significant loss of mass and structural failure.
Ensuring Pure Carbonization
Argon ensures the chemical reaction remains strictly pyrolytic (decomposition by heat) rather than oxidative (decomposition by burning).
This creates a controlled pathway for the polymer to transform into a pure pyrolytic carbon structure. This purity is directly responsible for the superior mechanical properties and physical integrity of the final product.
Protecting Functional Additives
Preserving Metallic States
In advanced applications where polymer precursors are doped with metal nanoparticles, such as copper, the argon atmosphere plays a dual role.
It protects the metal from oxidizing into metal oxides (e.g., preventing copper from becoming copper oxide).
Maintaining Active Components
By strictly controlling the atmosphere, metals can be maintained in their zero-valent metallic state (Cu0).
As noted in specific filtration applications, keeping the metal in this zero-valent state is essential for its chemical activity, such as the efficient removal of iodine from solutions.
Common Pitfalls in Atmosphere Control
The Danger of Impurities
Using argon that is not "high purity" can introduce trace amounts of oxygen or moisture into the furnace.
Even microscopic amounts of oxygen can cause surface defects or weak points in the pyrolytic carbon structure, compromising the material's final strength.
Flow Rate Consistency
Simply introducing argon is not enough; a continuous, positive flow is required.
If the flow rate drops, or if the furnace is not properly sealed, atmospheric oxygen can back-diffuse into the chamber. This leads to localized oxidation, resulting in inconsistent material properties across the sample.
Making the Right Choice for Your Goal
To maximize the quality of your pyrolysis process, align your atmospheric controls with your specific material objectives:
- If your primary focus is Structural Integrity: Ensure the argon purge is thorough before heating begins to prevent initial oxidative damage to the carbon backbone.
- If your primary focus is Chemical Functionality (e.g., Catalysis): Use the highest purity argon available to strictly maintain the zero-valent state of any metallic nanoparticles embedded in the precursor.
High-purity argon is not just a carrier gas; it is the fundamental boundary condition that makes the creation of high-quality pyrolytic carbon possible.
Summary Table:
| Feature | Role of High-Purity Argon | Impact on Material |
|---|---|---|
| Environment | Displaces oxygen and moisture | Prevents oxidative combustion |
| Chemical State | Maintains zero-valent metallic states | Preserves catalytic/functional activity |
| Mass Balance | Inhibits CO/CO2 formation | Minimizes mass loss and structural defects |
| Purity | Provides inert chemical shield | Ensures high-quality pyrolytic carbon structure |
Elevate Your Material Research with KINTEK Precision
Don't let atmospheric impurities compromise your carbonization results. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems designed for rigorous atmosphere control. Whether you need to maintain zero-valent metallic states or ensure structural integrity in polymer pyrolysis, our customizable lab high-temperature furnaces provide the stability your research demands.
Ready to optimize your thermal processing? Contact our technical team today to discuss your unique application needs!
Visual Guide
References
- Ali Naderi, Yeqing Wang. Stiff, lightweight, and programmable architectured pyrolytic carbon lattices via modular assembling. DOI: 10.1038/s43246-025-00739-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What heating element is used in a multi station vacuum tube furnace and what types of furnace tubes can be used? Optimize Your High-Temp Processes
- What function does a tube furnace serve in converting electrospun fibers into CNFs? Mastering the Carbonization Path
- How does a tube furnace facilitate the transformation of natural wood into a Carbonized Wood carrier? Master Pyrolysis
- What is the primary benefit of horizontal tube furnaces? Achieve Superior Thermal Uniformity for Your Materials
- What is the function of a high-temperature tube furnace in ZIF-8 carbonization? Achieve High-Performance NC Supports
- How are tube furnaces utilized in nanotechnology? Essential for Precise Nanomaterial Synthesis
- How does a vertical tube furnace achieve energy efficiency? Key Design Features for Lower Energy Costs
- What are the key heat treatment processes performed in horizontal furnaces? Master Annealing, Hardening, and More



















