The Self-Propagating High-Temperature Synthesis (SHS) reactor functions as a high-pressure containment vessel designed to convert titanium sponge into titanium hydride through a self-sustaining chemical reaction. Instead of acting as a traditional furnace that applies heat externally, the reactor creates a controlled hydrogen environment that allows the titanium to generate its own heat, driving the hydrogenation process internally.
Core Takeaway The SHS reactor leverages the exothermic nature of the titanium-hydrogen reaction to drive the process, rather than relying on continuous external energy. It facilitates a "combustion wave" that propagates through the material, rapidly transforming ductile titanium into brittle titanium hydride suitable for powder production.
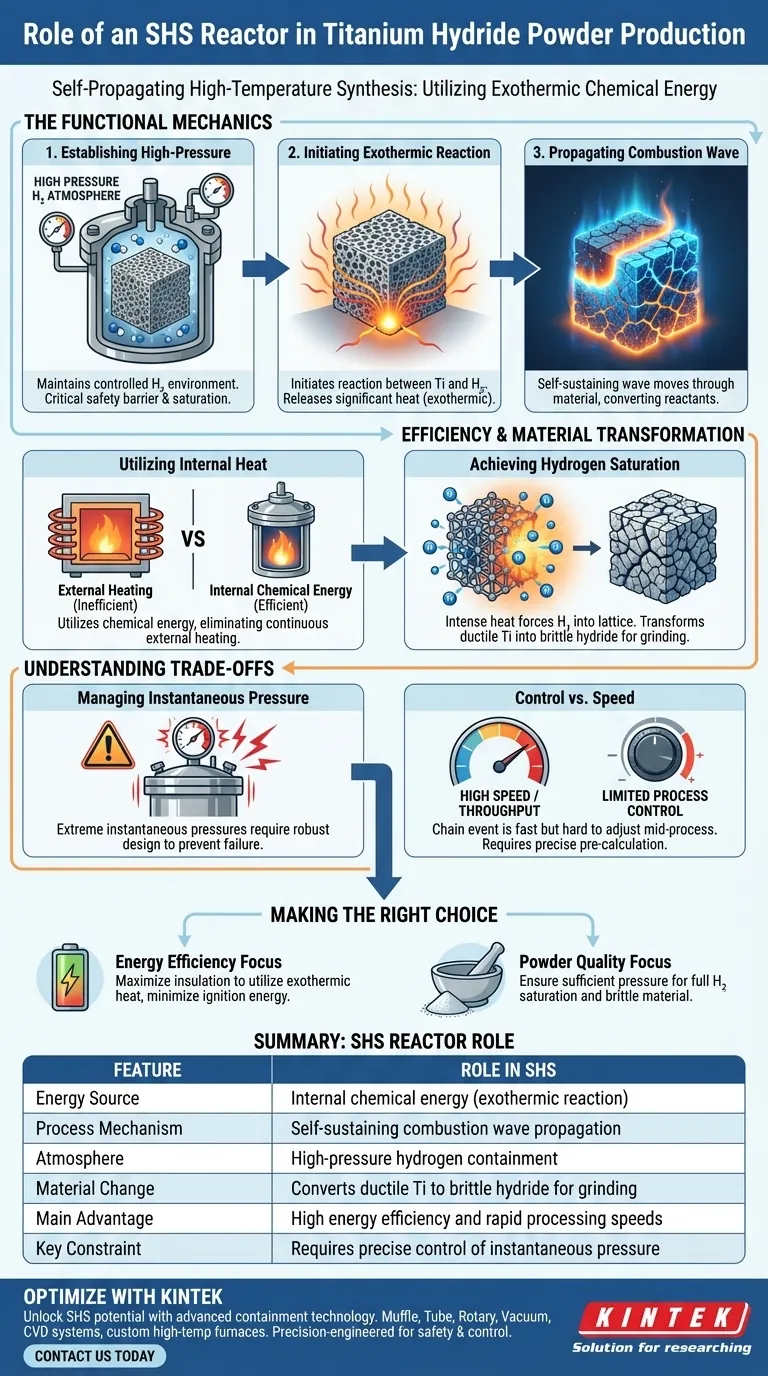
The Functional Mechanics of the Reactor
Establishing the High-Pressure Environment
The primary role of the reactor is to maintain a controlled, high-pressure hydrogen atmosphere.
This pressurized environment is essential for initiating the reaction between the gas and the solid titanium sponge. The vessel acts as a critical safety barrier, containing the reactants while allowing for the necessary saturation levels to occur.
Initiating the Exothermic Reaction
Once the environment is pressurized, the reactor system initiates the chemical interaction between the titanium powder and hydrogen.
This interaction is exothermic, meaning it releases a significant amount of heat. The reactor is designed to harness this release rather than suppress it, using the energy to fuel the next stage of the process.
Propagating the Combustion Wave
The defining feature of SHS technology is the combustion wave.
Instead of heating the entire batch simultaneously from the outside, the reaction starts at a specific point and travels through the titanium compact as a wave. The reactor design ensures this wave moves stably through the material, converting reactants as it passes.
Efficiency and Material Transformation
Utilizing Internal Heat
The SHS reactor allows for distinct energy efficiency by utilizing chemical energy instead of electrical heating.
Once the reaction is triggered, the heat released by the formation of titanium hydride is sufficient to sustain the process. This eliminates the need for continuous external heating, distinguishing it from conventional sintering or diffusion methods.
Achieving Hydrogen Saturation
The ultimate goal of the reactor is to achieve high levels of hydrogen saturation within the titanium lattice.
The intense, localized heat of the combustion wave forces hydrogen into the metal structure. This saturation transforms the naturally ductile titanium into a brittle hydride phase, which is the physical property required to easily grind the material into a fine powder later.
Understanding the Trade-offs
Managing Instantaneous Pressure
While efficient, the SHS process generates extreme internal conditions.
The reactor must be robust enough to withstand extreme instantaneous pressures caused by the rapid release of energy. Failure to contain these pressure spikes can lead to equipment damage or safety hazards.
Control vs. Speed
The speed of the self-propagating wave offers high throughput, but it presents a challenge in process control.
Unlike a slow-heating furnace where temperature can be adjusted gradually, the SHS reaction is a chain event. The reactor parameters (initial pressure and reactant density) must be calculated precisely beforehand, as adjusting the "wave" mid-process is difficult.
Making the Right Choice for Your Goal
To maximize the effectiveness of an SHS reactor for titanium hydride production, align your operational parameters with your specific output requirements.
- If your primary focus is Energy Efficiency: Rely on the reactor's insulation to maximize the utilization of the exothermic heat, minimizing the initial ignition energy required.
- If your primary focus is Powder Quality: Ensure the reactor pressure is sufficient to drive full hydrogen saturation, as incomplete saturation will leave the titanium too ductile to grind effectively.
The SHS reactor is not merely a heating vessel; it is a precision pressure chamber that turns the chemical potential of titanium into the thermal energy required for its own transformation.
Summary Table:
| Feature | Role in SHS Reactor |
|---|---|
| Energy Source | Internal chemical energy (exothermic reaction) |
| Process Mechanism | Self-sustaining combustion wave propagation |
| Atmosphere | High-pressure hydrogen containment |
| Material Change | Converts ductile titanium to brittle hydride for grinding |
| Main Advantage | High energy efficiency and rapid processing speeds |
| Key Constraint | Requires precise control of instantaneous pressure |
Optimize Your Material Synthesis with KINTEK
Unlock the full potential of Self-Propagating High-Temperature Synthesis with advanced containment technology. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique material science needs.
Whether you are producing high-purity titanium hydride or exploring new exothermic chemical processes, our precision-engineered systems provide the safety and control required for high-pressure environments.
Ready to elevate your lab's capabilities? Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Н. П. Черезов, М. И. Алымов. SHS-Hydrogenation, Thermal Dehydrogenation, and Plasma Spheroidization to Produce Spherical Titanium Powders from Titanium Sponge. DOI: 10.3390/alloys3030014
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How is the protective atmosphere box furnace applied in metal heat treatment? Enhance Metal Properties with Controlled Atmospheres
- What types of gases are commonly used in atmosphere furnaces and why? Optimize Your Heat Treatment Process
- What are the thermal insulation properties of argon in furnace applications? Unlock Material Purity and Efficiency
- What are the key features of calcining furnaces? Boost Efficiency and Quality in Material Processing
- What are the main components of a program-controlled atmosphere furnace? Unlock Precision in Thermal Processing
- What types of gases are used in inert ovens to create a controlled environment? Discover Nitrogen vs. Argon for Optimal Results
- How does an atmosphere protection furnace ensure the quality of CoCrFeNiMn coatings? Optimized Heat Treatment Solutions
- Why must Boron Nitride Spheres with binders undergo heat treatment in air? Unlock Maximum Thermal Conductivity



















