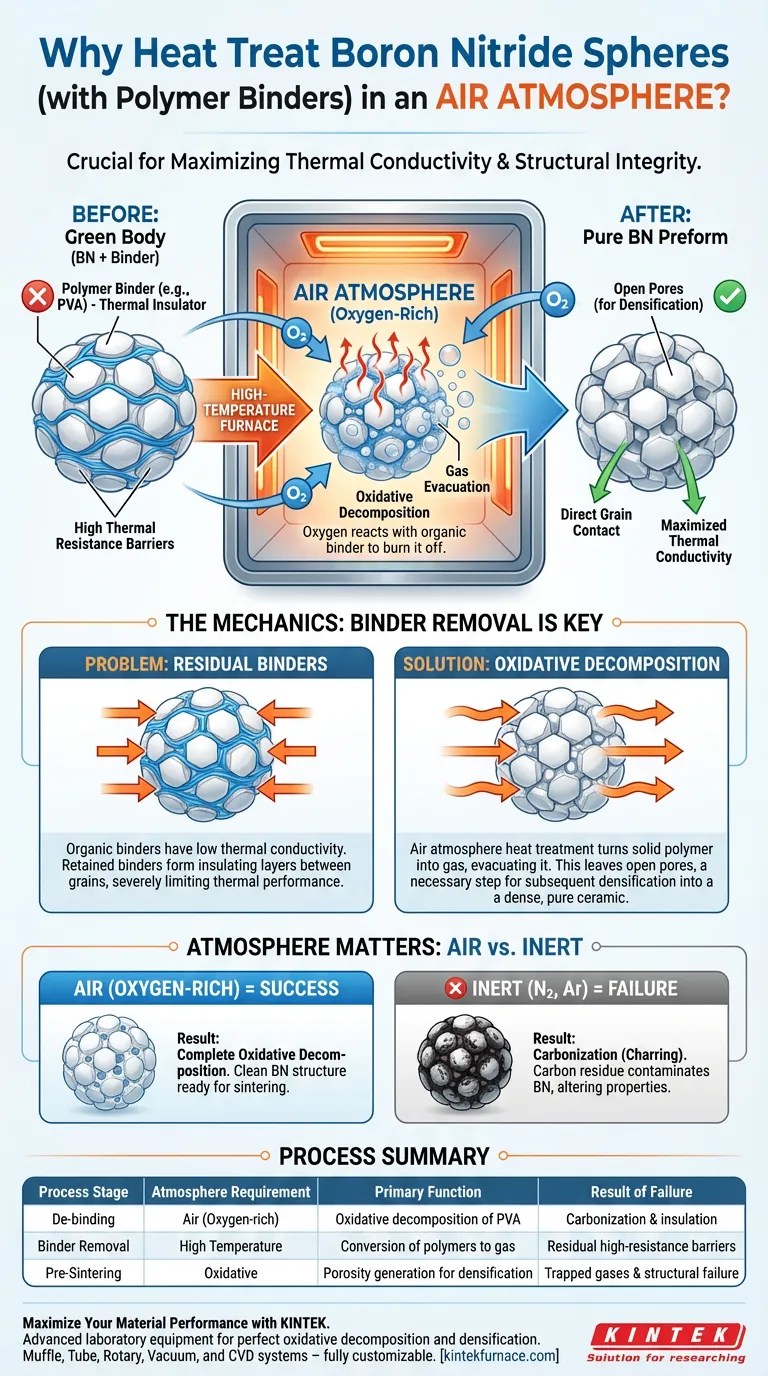
Boron Nitride Spheres containing polymer binders must undergo heat treatment in an air atmosphere to facilitate oxidative decomposition. This environment is essential to burn off organic components, such as Polyvinyl Alcohol (PVA), which act as temporary binders. Without the presence of oxygen in the furnace, these binders cannot be effectively removed from the material matrix.
The primary goal of air-atmosphere heat treatment is to eliminate thermally insulating organic binders. If retained, these binders form high-resistance barriers between grains, severely limiting the intrinsic thermal conductivity of the Boron Nitride.

The Mechanics of Conductivity Preservation
The Necessity of Oxidative Decomposition
The polymer binders used in Boron Nitride Spheres are organic compounds. To remove them, the material must be subjected to high temperatures in an air atmosphere.
The oxygen in the air reacts with the organic binders, causing them to decompose oxidatively. This reaction effectively turns the solid polymer into gas, evacuating it from the sphere structure.
Preventing Thermal Resistance Layers
The core problem with polymer binders is their electrical and thermal behavior relative to Boron Nitride. These organic materials possess low thermal conductivity.
If the binder is not removed, it remains situated between the individual Boron Nitride grains. This creates a "high thermal resistance layer," acting as an insulator that prevents heat from moving efficiently from one grain to the next.
Preparing for Densification
The removal of the binder is a preparatory step for the final structural hardening of the material. As the binder burns away, it leaves behind open pores within the spheres.
These pores are not permanent defects; rather, they are necessary voids that allow the material to be densified during subsequent high-temperature sintering steps. You cannot achieve a dense, pure ceramic structure if the space is still occupied by residual polymer.
Understanding the Trade-offs
Porosity Generation
The immediate result of this heat treatment is an increase in porosity. By removing the binder, you are physically removing volume from the sphere, leaving empty space.
While this lowers the density temporarily, it is a required trade-off to ensure chemical purity. Attempting to sinter without this porous, binder-free state would likely lead to trapped gases and structural failure.
Atmosphere Sensitivity
The requirement for an air atmosphere is specific and non-negotiable for this stage. Using an inert atmosphere (like nitrogen or argon) during this specific de-binding phase would fail to oxidize the PVA.
This would result in carbonization (charring) of the binder rather than removal. Carbon residues would contaminate the Boron Nitride, permanently altering its thermal and electrical properties.
Optimizing Your Thermal Management Strategy
The heat treatment process is a balance between removing impurities and preparing the structure for final densification.
- If your primary focus is maximum thermal conductivity: Ensure the heat treatment fully oxidizes the binder to eliminate any insulating layers between Boron Nitride grains.
- If your primary focus is structural density: View the air heat treatment as a critical preparatory step that clears the way for effective high-temperature sintering.
By ensuring the complete oxidative decomposition of polymer binders, you unlock the full thermal potential of the Boron Nitride material.
Summary Table:
| Process Stage | Atmosphere Requirement | Primary Function | Result of Failure |
|---|---|---|---|
| De-binding | Air (Oxygen-rich) | Oxidative decomposition of PVA/binders | Carbonization and thermal insulation |
| Binder Removal | High Temperature | Conversion of polymers to gas | Residual high-resistance barriers |
| Pre-Sintering | Oxidative | Porosity generation for densification | Trapped gases and structural failure |
Maximize Your Material Performance with KINTEK
Precise thermal management is the difference between material failure and peak performance. KINTEK provides the advanced laboratory equipment necessary to achieve perfect oxidative decomposition and densification. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the specific heat treatment requirements of your Boron Nitride and advanced ceramic projects.
Don't let residual binders compromise your thermal conductivity. Contact KINTEK today to find the ideal high-temperature furnace solution for your lab.
Visual Guide
References
- Hongbo Jiang, Ying Chen. Unleashing the Potential of Boron Nitride Spheres for High‐Performance Thermal Management. DOI: 10.1002/cnma.202300601
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
People Also Ask
- What type of vacuum pumps are used in low vacuum atmosphere furnaces? Reliable Rotary Vane Pumps for Cost-Effective Heating
- What are the working principles and gas environments of box furnaces and atmosphere furnaces? Choose the Right Furnace for Your Lab
- How does the controlled thermal environment of a laboratory furnace support the hydrothermal synthesis of NH2-MIL-125?
- Why is stress relief annealing essential for SLM titanium scaffolds? Ensure Durability and Fatigue Resistance
- What role do atmosphere furnaces play in new energy material R&D? Unlock Precision Synthesis for Batteries and Solar Cells
- What are the four main types of controlled atmospheres used in these furnaces? Optimize Your Heat Treatment Processes
- What is the significance of the preheating step using a high-temperature furnace? Ensure Pellets Strength and Integrity
- Why is a rotameter essential for controlling the atmosphere within an oily sludge pyrolysis reactor? Master Gas Flow Control



















