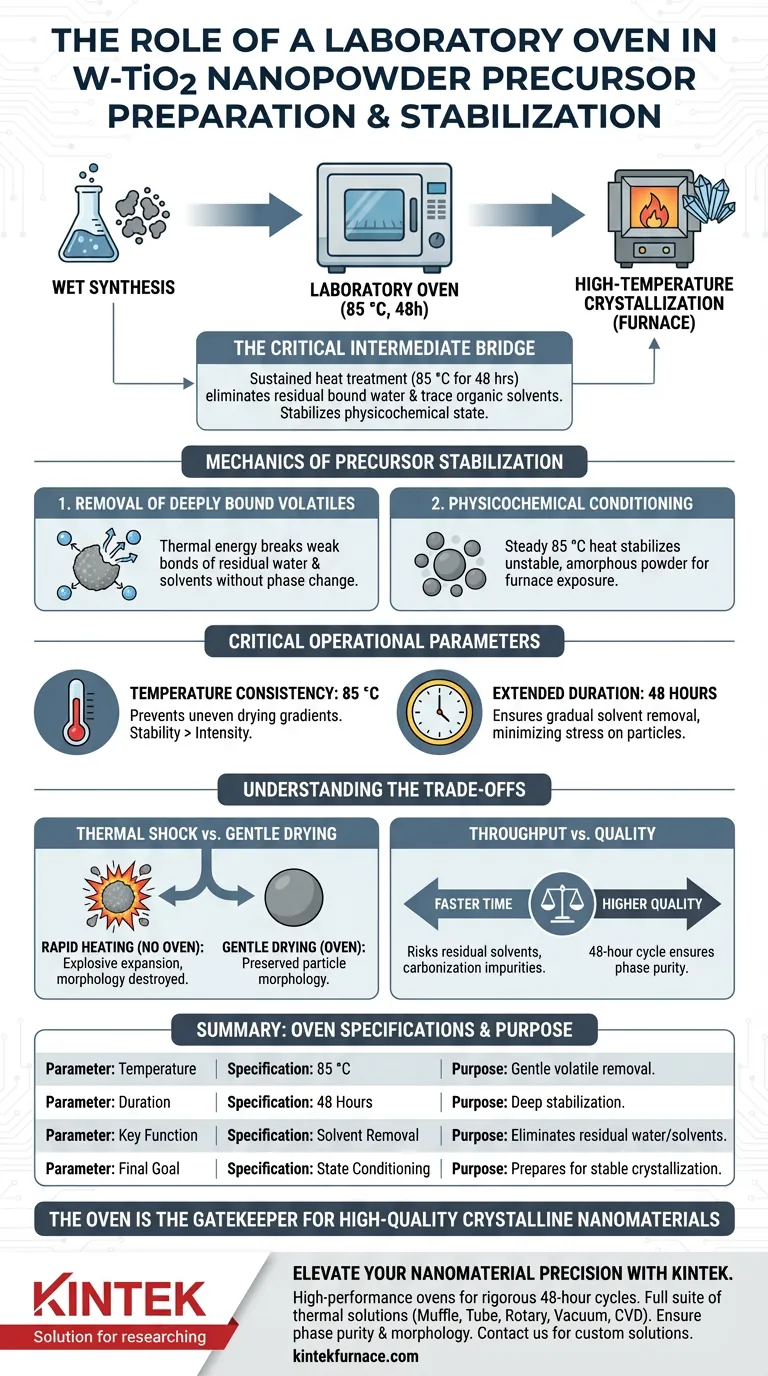
A laboratory oven serves as the critical intermediate bridge between wet synthesis and high-temperature crystallization for W-doped Titanium Dioxide (W-TiO2) precursors. It functions to perform a sustained heat treatment on amorphous powders, typically maintaining a constant temperature of 85 °C for up to 48 hours. This controlled environment effectively eliminates residual bound water and trace organic solvents, stabilizing the material's physicochemical state before it enters a furnace.
Core Takeaway The laboratory oven does not just dry the material; it stabilizes the precursor's chemical structure. By gently removing volatiles at moderate temperatures, it ensures the amorphous powder is chemically "quiet" and physically prepared for the harsh conditions of high-temperature calcination.
The Mechanics of Precursor Stabilization
Removal of Deeply Bound Volatiles
The primary function of the oven in this specific application is the removal of stubborn impurities.
While initial filtration removes bulk liquid, the amorphous powder still retains residual bound water and trace organic solvents.
The oven provides the thermal energy required to break these weak bonds without initiating a phase change in the material itself.
Physicochemical Conditioning
The powder entering the oven is in an unstable, amorphous state.
By subjecting the powder to a steady 85 °C heat treatment, the oven stabilizes the physicochemical state of the precursor.
This "conditioning" phase prevents the material from reacting unpredictably when later exposed to the extreme heat of a furnace.
Critical Operational Parameters
Temperature Consistency
For W-TiO2 precursors, temperature stability is more critical than temperature intensity.
The process relies on maintaining a constant environment, cited specifically as 85 °C in standard protocols.
Fluctuations in temperature could lead to uneven drying gradients within the powder bed.
Extended Duration
The stabilization process is not instantaneous.
Protocols require significant time, often spanning 48 hours.
This slow, extended duration ensures that the removal of solvents occurs gradually, minimizing stress on the particle structure.
Understanding the Trade-offs
Thermal Shock vs. Gentle Drying
One might be tempted to skip the oven and place the precursor directly into a high-temperature furnace.
However, rapid heating causes trapped moisture and solvents to expand explosively on a microscopic level.
This can destroy the particle morphology or lead to severe agglomeration, rendering the nanopowder useless for high-performance applications.
Throughput vs. Quality
The 48-hour oven cycle creates a bottleneck in production speed.
Reducing this time increases throughput but risks leaving residual organic solvents in the core of the material.
If these solvents remain during calcination, they can carbonize, introducing impurities that degrade the optical or electronic properties of the final W-TiO2 product.
Making the Right Choice for Your Goal
To optimize your W-TiO2 preparation, align your oven usage with your specific processing targets:
- If your primary focus is Phase Purity: Ensure the full 48-hour cycle is completed at 85 °C to guarantee all organic solvents are removed prior to calcination.
- If your primary focus is Particle Morphology: Avoid increasing the oven temperature to speed up drying, as higher ramp rates can induce agglomeration in the amorphous powder.
The oven is not merely a dryer; it is the gatekeeper that determines whether your precursor survives the transition to a high-quality crystalline nanomaterial.
Summary Table:
| Parameter | Specification | Purpose in W-TiO2 Preparation |
|---|---|---|
| Temperature | 85 °C | Gentle removal of volatiles without phase change |
| Duration | 48 Hours | Ensures deep stabilization and gradient-free drying |
| Key Function | Solvent Removal | Eliminates residual water and organic solvents |
| Final Goal | State Conditioning | Prepares amorphous powder for stable crystallization |
Elevate Your Nanomaterial Precision with KINTEK
Don’t let improper drying compromise your research. KINTEK provides high-performance laboratory ovens designed for the rigorous 48-hour stability cycles required for W-TiO2 and other sensitive precursors.
Backed by expert R&D and world-class manufacturing, we offer a full suite of thermal solutions—including Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your unique lab requirements. Ensure phase purity and perfect particle morphology by choosing equipment built for consistency.
Ready to optimize your synthesis workflow? Contact us today to find your custom furnace solution.
Visual Guide
References
- Khley Cheng, Andreï Kanaev. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. DOI: 10.3390/nano14090765
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the primary function of the low-temperature pyrolysis process? Ensure Safe Battery Recycling with Pretreatment
- Why is precise nitrogen flow critical for AlN nanofibers? Mastering High-Performance Nitridation Results
- What are the advantages of using a corundum crucible with a graphite sleeve in AlV55 alloy smelting? Ensure Pure Alloys
- How do surface oxidation systems improve the interface performance of graphitized fibers? Maximize Composite Strength
- How is the thermal stability of KBaBi compounds evaluated? Discover Precise XRD & Heat Treatment Limits
- How does the addition of RhCl3 facilitate the synthesis of RhSeCl crystals? Unlock High-Quality Crystal Growth
- What are the equipment requirements for high-temperature furnaces during magnetic biochar synthesis? Find the key specs.
- What is the function of an industrial drying oven in PET waste pretreatment? Optimize Your Activated Carbon Production



















