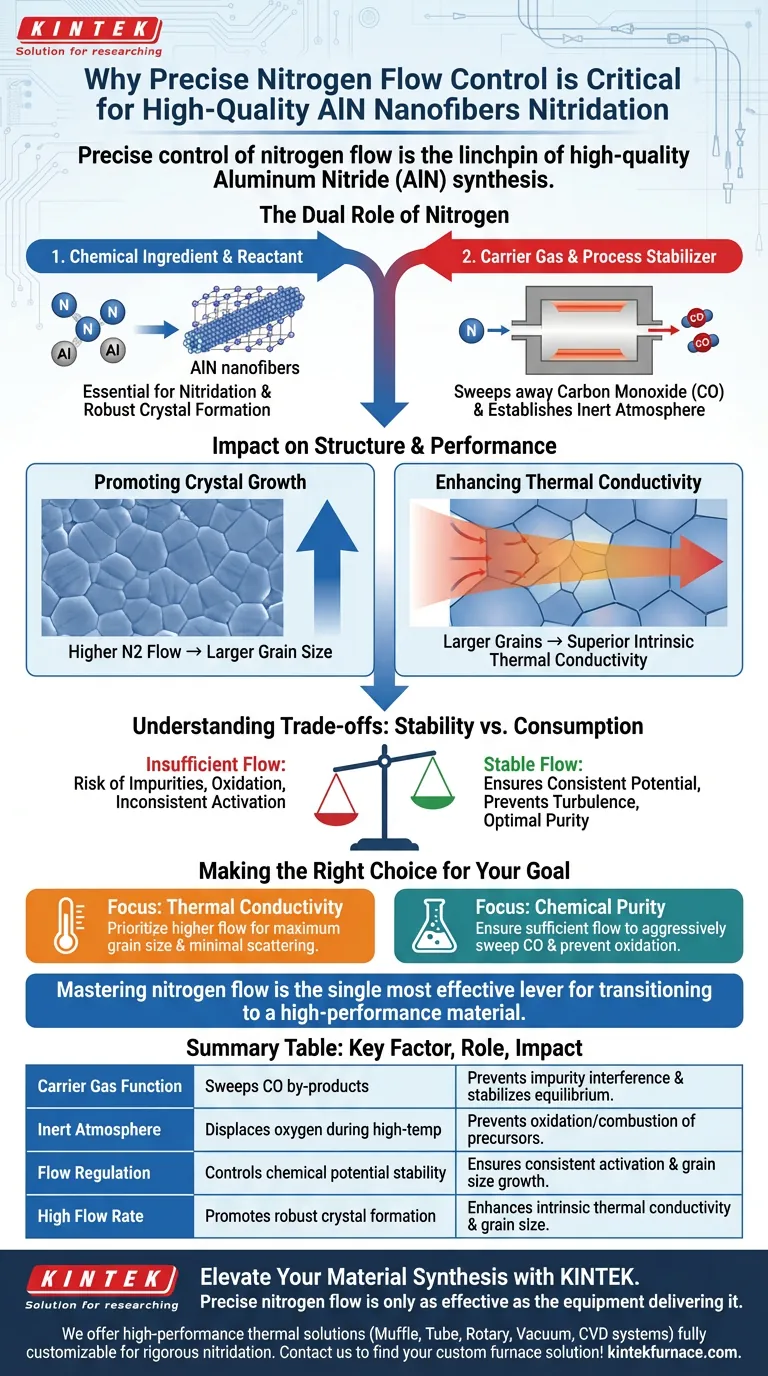
Precise control of nitrogen flow is the linchpin of high-quality Aluminum Nitride (AlN) synthesis. It serves a dual purpose: acting as the vital reactant for nitridation while simultaneously functioning as a carrier gas to sweep away volatile by-products like carbon monoxide. Without specific flow regulation, you compromise the reaction environment, resulting in material oxidation, poor crystal structure, and degraded thermal properties.
Regulating nitrogen flow is not just about supplying a reactant; it is the primary mechanism for purging impurities and driving crystal growth. Optimizing this flow directly correlates to larger grain sizes and superior thermal conductivity in the final nanofiber application.

The Dual Role of Nitrogen
To understand why flow rate is critical, you must view nitrogen as both a chemical ingredient and a process stabilizer.
Acting as a Carrier Gas
During the nitridation reaction, volatile by-products—specifically carbon monoxide (CO)—are generated.
Nitrogen acts as a sweeping agent, efficiently removing these gases from the reaction zone. If CO is allowed to linger, it can interfere with the reaction equilibrium and introduce impurities.
Establishing the Inert Atmosphere
The flow of nitrogen creates a strictly inert environment within the furnace.
This is essential for preventing the oxidation or combustion of precursors (such as hydrochar) at high temperatures. By displacing oxygen, the nitrogen flow protects the material integrity during the vulnerable activation phases.
Impact on Material Structure and Performance
Beyond basic protection, the flow rate directly dictates the physical quality of the nanofibers.
Promoting Crystal Growth
There is a direct relationship between nitrogen flow rates and the morphology of the AlN nanofibers.
A maintained, high nitrogen flow rate has been shown to significantly enlarge the grain size of the material. This flow promotes the optimal conditions necessary for robust crystal formation.
Enhancing Thermal Conductivity
The ultimate goal of synthesizing AlN nanofibers is often to leverage their intrinsic thermal properties.
Because higher flow rates lead to larger grain sizes, they consequently enhance the intrinsic thermal conductivity of the resulting nanofibers. Precise control allows you to tune the material for maximum thermal performance.
Understanding the Trade-offs
While high flow is generally beneficial, the key word is "control."
The Risk of Insufficient Flow
If the flow rate drops below the critical threshold, the removal of gaseous by-products becomes inefficient.
This stagnation creates an unstable chemical potential in the reaction zone, leading to inconsistent activation and potential contamination of the fiber surface.
Stability vs. Consumption
Maintaining a stable flow (e.g., 150 mL/min in specific contexts) ensures the chemical potential remains constant.
However, the system must be balanced to ensure the flow is sufficient to act as a carrier without being wasteful or causing turbulence that could disturb the nanofiber formation.
Making the Right Choice for Your Goal
When setting your process parameters, align your nitrogen flow strategy with your specific material requirements.
- If your primary focus is Thermal Conductivity: Prioritize higher nitrogen flow rates to maximize grain size and minimize phonon scattering boundaries.
- If your primary focus is Chemical Purity: Ensure the flow rate is sufficient to aggressively sweep carbon monoxide and prevent any oxidation of the precursor materials.
Mastering the nitrogen flow rate is the single most effective lever for transitioning from a functional AlN material to a high-performance one.
Summary Table:
| Key Factor | Role in Nitridation Process | Impact on AlN Nanofibers |
|---|---|---|
| Carrier Gas Function | Sweeps away volatile CO by-products | Prevents impurity interference and stabilizes equilibrium |
| Inert Atmosphere | Displaces oxygen during high-temp phases | Prevents oxidation/combustion of precursors |
| Flow Regulation | Controls chemical potential stability | Ensures consistent activation and grain size growth |
| High Flow Rate | Promotes robust crystal formation | Enhances intrinsic thermal conductivity and grain size |
Elevate Your Material Synthesis with KINTEK
Precise nitrogen flow is only as effective as the equipment delivering it. At KINTEK, we empower researchers and manufacturers with high-performance thermal solutions designed for rigorous nitridation processes.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory or production needs. Whether you are aiming for maximum thermal conductivity or superior chemical purity in your AlN nanofibers, our advanced furnaces provide the stability and control you require.
Ready to optimize your high-temperature reactions? Contact us today to find your custom furnace solution!
Visual Guide
References
- Md. Shakhawat Hossain, Koji Nakane. Enhancing heat dissipation in polyurethane sheets through the incorporation of freeze‐dried aluminum nitride nanofiber. DOI: 10.1111/ijac.14725
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What are advanced materials and composites? Unlock Superior Performance for Your Innovations
- What role does a pyrolysis device play in the synthesis of porous carbon for supercapacitors? Essential Thermal Secrets
- What role does a laboratory drying oven play in the formation of polymer colloidal crystal templates? Mastering 3DOM Foundations
- What is the primary purpose of using nano-magnesium oxide as a template? Optimize Sulfur-Doped Porous Carbon Synthesis
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- How does the introduction of SiO2 as an additive improve the sintering process of solid electrolytes? Boost Densification
- How does a blast drying oven support the preparation of rubidium-doped mesoporous bioactive glass? Optimized Synthesis
- What is the significance of using a vacuum drying oven? Optimize Supercapacitor Electrode Performance



















