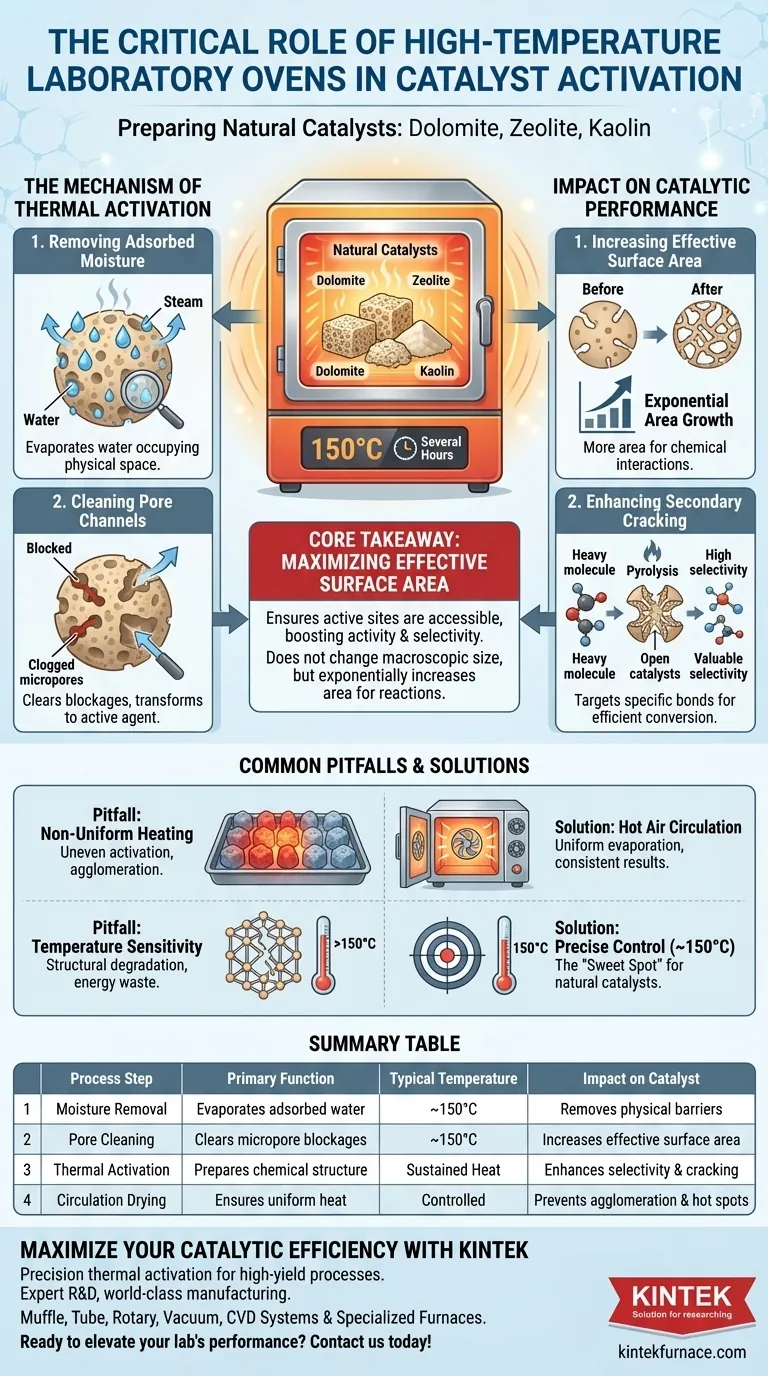
High-temperature laboratory ovens serve as the critical activation stage for natural catalysts like dolomite, zeolite, and kaolin. By subjecting these materials to sustained heat—typically around 150°C for several hours—the oven drives out adsorbed moisture and chemically prepares the internal structure of the material. This step is a prerequisite for ensuring the catalyst functions correctly during the secondary cracking stage of pyrolysis.
Core Takeaway The fundamental purpose of this thermal treatment is to maximize the effective surface area of the catalyst. By thoroughly cleaning pore channels of moisture and impurities, the oven ensures that the active sites within the catalyst are accessible, directly boosting catalytic activity and reaction selectivity.

The Mechanism of Thermal Activation
Removing Adsorbed Moisture
Natural mineral catalysts are porous and naturally attract water from the environment. This "adsorbed moisture" occupies the physical space within the catalyst's structure.
The laboratory oven provides a controlled thermal environment to evaporate this moisture. Without this step, the water molecules would act as a physical barrier, preventing reactants from entering the catalyst.
Cleaning Pore Channels
Beyond simple drying, the heat treatment functions as a deep cleaning process for the catalyst's micropores.
By maintaining a temperature of approximately 150°C, the process clears blockages within the pore channels. This transforms the material from a dormant mineral into an active chemical agent ready for interaction.
Impact on Catalytic Performance
Increasing Effective Surface Area
Catalysis is a surface-phenomenon game; the more surface area available, the more efficient the reaction.
The oven treatment does not change the macroscopic size of the catalyst, but it significantly increases the effective surface area. By unclogging the internal network of micropores, the total area available for chemical reactions increases exponentially.
Enhancing Secondary Cracking
For processes like pyrolysis, the goal is often "secondary cracking"—breaking down heavy molecules into lighter, more valuable ones.
A properly activated catalyst has high selectivity, meaning it targets specific chemical bonds. The oven-treated catalyst allows these heavy molecules to penetrate deeper into the pore structure, facilitating more efficient conversion into desired end products.
Common Pitfalls and Trade-offs
The Risk of Non-Uniform Heating
While the primary goal is activation, how that heat is applied matters. Inconsistent heating can lead to uneven activation, where some parts of the catalyst batch are ready while others remain dormant.
Advanced drying ovens often utilize hot air circulation. This ensures that moisture evaporation is uniform across the entire batch, preventing the migration or agglomeration of particles that can occur if drying is uneven or too rapid.
Temperature Sensitivity
More heat is not always better. While high-temperature furnaces are used for synthesizing single-atom catalysts or decomposing organic ligands at much higher temperatures, natural catalysts have a "sweet spot."
For materials like zeolite and dolomite in this context, the target is around 150°C. Exceeding necessary temperatures without cause can lead to unnecessary energy consumption or, in extreme cases, structural changes that might degrade the mineral's natural lattice.
Making the Right Choice for Your Goal
To maximize the efficiency of your catalyst preparation, align your oven settings with your specific objectives:
- If your primary focus is Maximizing Reactivity: Ensure the catalyst undergoes the full duration of treatment (several hours) at 150°C to guarantee all micropores are completely cleared of moisture.
- If your primary focus is Consistency: Prioritize ovens with active air circulation to ensure the entire batch dries uniformly, preventing "hot spots" or uneven activation.
The difference between a mediocre reaction and a high-yield process often lies in the precision of this initial thermal activation step.
Summary Table:
| Process Step | Primary Function | Typical Temperature | Impact on Catalyst |
|---|---|---|---|
| Moisture Removal | Evaporates adsorbed water | ~150°C | Removes physical barriers for reactants |
| Pore Cleaning | Clears micropore blockages | ~150°C | Increases effective surface area exponentially |
| Thermal Activation | Prepares chemical structure | Sustained Heat | Enhances selectivity and secondary cracking |
| Circulation Drying | Ensures uniform heat | Controlled | Prevents particle agglomeration and hot spots |
Maximize Your Catalytic Efficiency with KINTEK
Precision thermal activation is the difference between a mediocre reaction and a high-yield process. At KINTEK, we empower researchers and industrial labs with cutting-edge heating solutions designed for accuracy and uniformity.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized lab high-temperature furnaces—all fully customizable to your unique catalyst preparation needs. Whether you are working with dolomite, zeolite, or advanced synthetic catalysts, our systems ensure consistent pore cleaning and moisture removal for superior reactivity.
Ready to elevate your lab's performance? Contact us today to find your perfect thermal solution!
Visual Guide
References
- Indra Mamad Gandidi, Arinal Hamni. Integrated two-step co-pyrolysis under several low-cost natural catalysts to produce aromatic-rich liquid fuel from mixed municipal solid waste. DOI: 10.1093/ce/zkae092
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the defining characteristic of a muffle furnace? Achieve Pure, Uniform Heating for Your Lab
- What is the difference between a muffle furnace and an oven? A Guide to High-Temperature Processing Purity
- Why is a laboratory box resistance furnace essential for simulating long-term high-temperature oxidation exposure?
- What are the advantages of using a Microwave Muffle Furnace? Faster, Higher-Quality Activated Carbon Preparation
- What is the purpose of using a muffle furnace for LDH calcination? Unlock the Memory Effect for Advanced Reconstruction
- How is a high-temperature box furnace utilized during the calcination and sintering stages of SrVO3 precursors?
- What are the key disadvantages of a muffle furnace? Slow cycles, high energy use, and maintenance challenges
- What is the purpose of using a muffle furnace to fire Al2O3 ceramic shells at 1050°C? Enhance Strength and Purity



















