A laboratory box resistance furnace is the cornerstone of accurate high-temperature durability testing. It provides a strictly controlled, stable, static air environment that mimics the harsh operational realities of high-performance components, such as aircraft engine parts. By maintaining constant temperatures for durations extending up to 1000 hours, it allows engineers to observe slow-acting degradation processes that short-term testing would miss.
By facilitating long-term, constant-temperature exposure, this equipment transforms abstract material data into concrete evidence regarding oxide growth and structural integrity, enabling precise predictions of component failure.

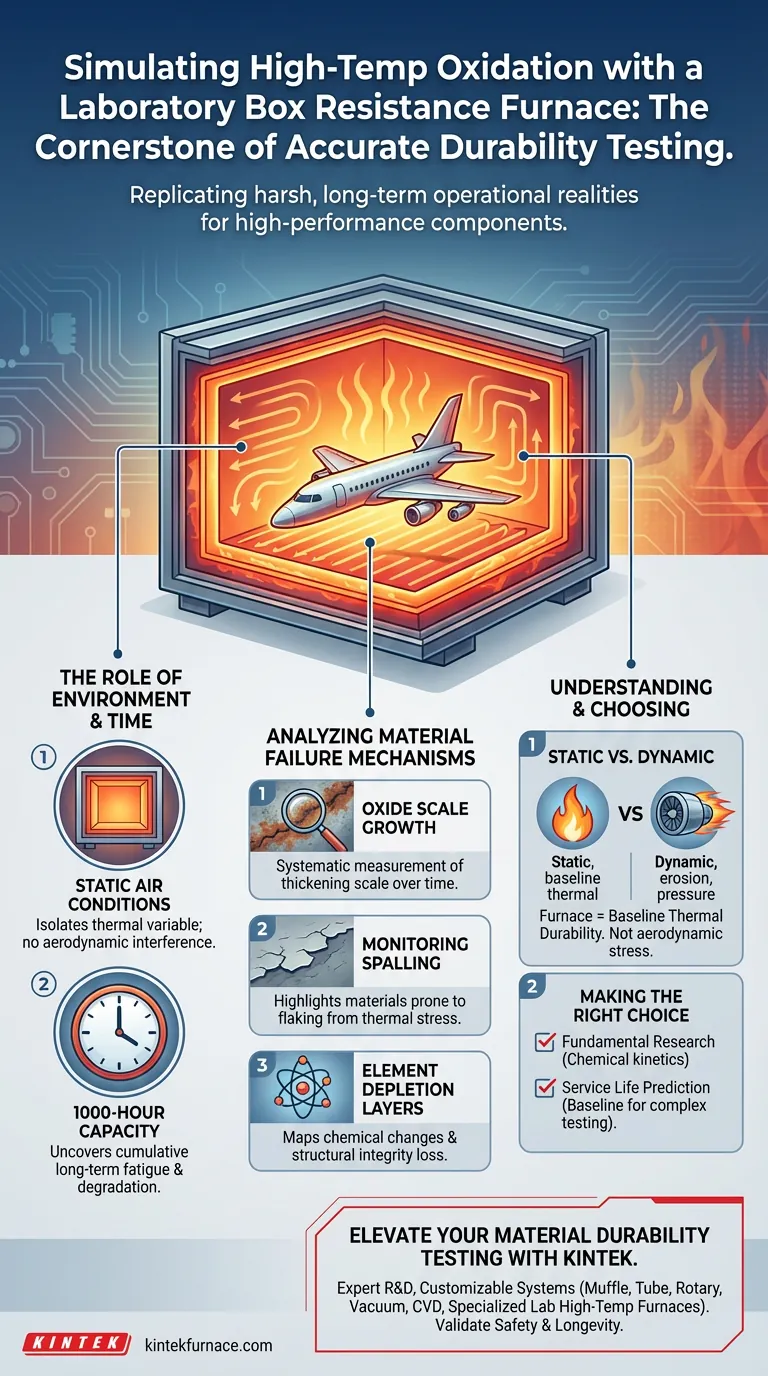
The Role of Environment and Time
Replicating Static Air Conditions
The primary function of the box resistance furnace is to create a "static air" environment. Unlike dynamic tests that introduce high-velocity airflow, this furnace isolates the variable of thermal exposure.
This stability allows researchers to focus exclusively on how the material reacts to heat in an oxygenated atmosphere without the interference of aerodynamic forces.
The Necessity of Long-Duration Testing
Material failure in high-performance engines rarely happens instantly; it is a cumulative process. The furnace is designed to sustain operations for up to 1000 hours.
This extended duration is critical for uncovering long-term fatigue and degradation patterns. It bridges the gap between theoretical material properties and actual service life expectancy.
Analyzing Mechanisms of Material Failure
Investigating Oxide Scale Growth
High temperatures cause materials to react with oxygen, forming an oxide scale on the surface. The furnace enables the systematic measurement of how this scale thickens over time.
Understanding the growth rate of this scale is vital for determining when a component will lose its dimensional tolerance or thermal conductivity.
Monitoring Spalling Tendencies
"Spalling" occurs when the protective or oxidized layers of a material flake off due to thermal stress. The constant-temperature environment highlights materials that are prone to this specific type of mechanical failure.
By identifying spalling tendencies early, engineers can predict the likelihood of debris generation within sensitive engine systems.
Tracking Element Depletion Layers
Over time, high heat causes specific elements within an alloy to diffuse or evaporate, altering the material's chemical composition. This creates "depletion layers" where the material becomes weaker.
The furnace facilitates the evolution of these layers, allowing scientists to map exactly when and how the material loses its structural integrity.
Understanding the Trade-offs
Static vs. Dynamic Simulation
While essential for chemical and thermal analysis, it is important to recognize the limitations of a "static air" environment.
This furnace simulates heat and oxidation, but it does not replicate the high-velocity erosion or mechanical pressures found in a live aircraft engine. Therefore, data derived here should be viewed as a baseline for thermal durability, rather than a complete simulation of aerodynamic stress.
Making the Right Choice for Your Goal
When designing your testing protocol, consider exactly which failure mode you are trying to isolate.
- If your primary focus is Fundamental Material Research: Use this furnace to define the chemical kinetics of oxide scale growth and element depletion without mechanical interference.
- If your primary focus is Service Life Prediction: Use the 1000-hour exposure capability to establish a baseline for durability before moving to more complex, dynamic rig testing.
The laboratory box resistance furnace provides the isolated, high-heat baseline required to validate the safety and longevity of critical aerospace components.
Summary Table:
| Feature | Benefit in Oxidation Testing |
|---|---|
| Static Air Environment | Isolates thermal/chemical reactions from aerodynamic interference. |
| 1000-Hour Capacity | Captures long-term degradation and fatigue missing in short-term tests. |
| Oxide Scale Analysis | Facilitates precise measurement of growth rates and spalling tendencies. |
| Element Depletion | Allows mapping of chemical changes and structural integrity loss. |
Elevate Your Material Durability Testing with KINTEK
Precise oxidation modeling requires equipment that can withstand the rigors of long-term, high-temperature exposure. At KINTEK, we empower researchers and manufacturers with high-performance laboratory solutions designed for accuracy and endurance.
Our Value to You: Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temp furnaces. All our systems are fully customizable to meet your unique simulation requirements, ensuring your aerospace or industrial components are validated for safety and longevity.
Ready to transform abstract material data into concrete performance insights? Contact us today to discuss your custom furnace needs!
Visual Guide
References
- J. W. X. Wo, H.J. Stone. The Effect of Nb, Ta, and Ti on the Oxidation of a New Polycrystalline Ni-Based Superalloy. DOI: 10.1007/s11085-023-10218-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How do box type resistance furnaces contribute to catalytic material preparation? Unlock Precision in Catalyst Synthesis
- What safety features are enhanced in muffle furnaces? Discover Advanced Protection for Your Lab
- How does the built-in venting system in a muffle furnace improve performance? Boost Durability and Safety in Your Lab
- What are some advancements in modern muffle furnace technology? Boost Precision and Efficiency in Your Lab
- What is the mechanism for the furnace door in a muffle furnace? Discover the Double-Hinge Design for Perfect Sealing
- What is the typical function of a laboratory muffle furnace in the preparation of chemical catalysts? | KINTEK
- What role does an industrial microwave muffle furnace play in the sintering process of porous mullite ceramic skeletons?
- What is the role of an industrial-grade high-temperature box furnace in Ni-Ti-Hf-La alloy post-processing?



















