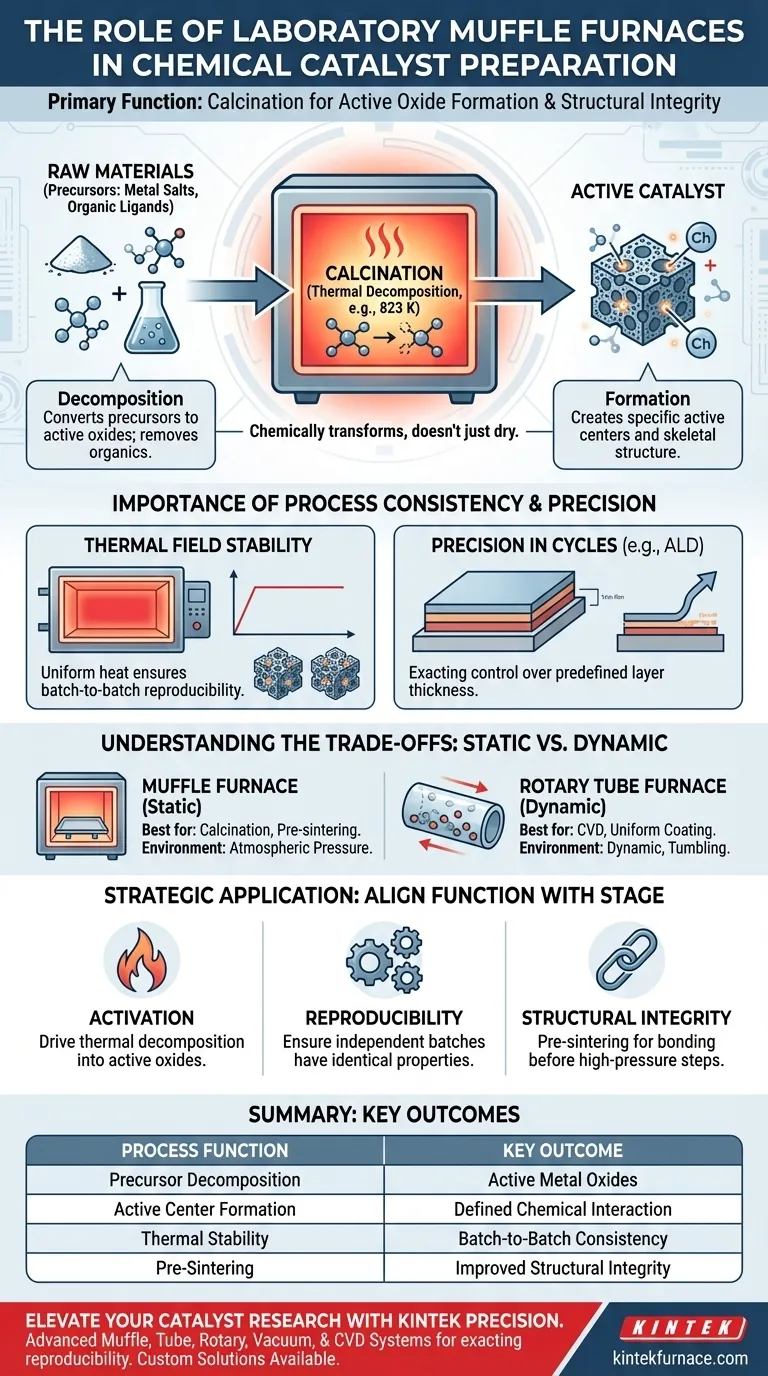
The primary function of a laboratory muffle furnace in chemical catalyst preparation is calcination. Through a preset temperature curve, the furnace heats raw materials to decompose metal salt precursors. This process chemically converts these precursors into active oxides and facilitates the initial formation of the catalyst's specific active centers and skeletal structure.
The muffle furnace does not simply dry materials; it chemically transforms them. Its ability to maintain a precise and stable thermal field is the defining factor in ensuring that every batch of catalysts possesses consistent structural integrity and chemical activity.

The Mechanics of Calcination
Decomposition of Precursors
The raw materials for catalysts often begin as metal salts or contain organic ligands. These components must be broken down to become chemically active.
The muffle furnace heats the support material (often around 823 K) to achieve thermal decomposition. This step effectively removes organic components and converts the metal elements into their respective active oxides.
Formation of Active Centers
Heat treatment is responsible for more than just chemical purity; it dictates the physical architecture of the material.
The calcination process initiates the formation of specific active centers. It creates the underlying skeletal structure that defines how the catalyst will interact with other chemicals in future applications.
The Importance of Process Consistency
Thermal Field Stability
In catalyst research and production, reproducibility is paramount. A minor fluctuation in temperature can alter the ratio of active oxides.
The laboratory muffle furnace provides excellent thermal field stability. This ensures that the heat is applied uniformly, guaranteeing the consistency and stability of the catalyst across different production batches.
Precision in Advanced Cycles
For complex preparations involving Atomic Layer Deposition (ALD), the furnace plays a cyclical role.
By alternating ALD cycles with muffle furnace calcination, researchers can precisely manage the weight gain of thin films (such as BaZrO3). This allows for exacting control over the final predefined thickness of the catalyst layers.
Understanding the Trade-offs
Static vs. Dynamic Processing
The muffle furnace typically heats materials in a static environment. This is ideal for calcination and pre-sintering compacts to improve structural integrity.
However, this differs from rotary tube furnaces, which are better suited for processes like Chemical Vapor Deposition (CVD). Rotary furnaces tumble the particles to ensure precursor gases uniformly coat every surface, a feature standard muffle furnaces lack.
Atmosphere and Pressure Limits
Muffle furnaces generally operate under atmospheric pressure.
While effective for preliminary bonding and calcination, they are not designed for densification processes that require high pressure, such as high-pressure hot re-pressing.
How to Apply This to Your Project
To maximize the utility of your laboratory furnace, align its function with your specific stage of preparation:
- If your primary focus is activation: Use the furnace to drive the thermal decomposition of precursors into active oxides.
- If your primary focus is reproducibility: Rely on the furnace's thermal stability to ensure that independent batches display identical catalytic properties.
- If your primary focus is structural integrity: Utilize the furnace for pre-sintering to bond powder particles before subjecting them to high-pressure densification.
By strictly controlling the thermal history of your material, the muffle furnace bridges the critical gap between raw chemical precursors and a high-performance active catalyst.
Summary Table:
| Process Function | Description | Key Outcome |
|---|---|---|
| Precursor Decomposition | Heating metal salts/organic ligands to specific temperatures (e.g., 823 K) | Conversion into active metal oxides |
| Active Center Formation | Creating the catalyst's skeletal structure through controlled heating | Defines future chemical interaction capability |
| Thermal Stability | Maintaining a precise and uniform thermal field | Ensures batch-to-batch consistency and activity |
| Pre-Sintering | Preliminary bonding of powder particles | Improved structural integrity for further processing |
Elevate Your Catalyst Research with KINTEK Precision
Consistency is the backbone of catalyst preparation. KINTEK’s advanced laboratory muffle furnaces provide the thermal field stability required to transform raw precursors into high-performance active oxides with exacting reproducibility.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique chemical and material science requirements. Whether you are scaling ALD processes or perfecting calcination cycles, our systems ensure your materials achieve their predefined structural and chemical targets.
Ready to optimize your lab’s thermal processing? Contact us today to find your custom solution!
Visual Guide
References
- Bhupendra Pratap Singh, Rajendra Srivastava. Catalytic Hydrogenation of Lignin Ethers and Bio‐Oil Using Non‐Noble Cobalt Catalysts. DOI: 10.1002/cssc.202402714
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a high-temperature muffle furnace facilitate the modification of dolomite? Engineering Superior Adsorbents
- What factors affect the price of muffle furnaces? Key Drivers for Smart Lab Investment
- Why is a high-precision muffle furnace required for BCZT xerogel pre-calcination? Ensure Pure Phase and Reactivity
- How do crucible furnaces facilitate quick alloy changes? Achieve Unmatched Production Flexibility
- How do box resistance furnaces facilitate the tempering process for quenched 60Si2CrV spring steel? Precision Hardening
- How does a muffle furnace achieve high temperatures with uniformity and accuracy? Discover the Design Secrets for Precise Heat Treatment
- How are high temperatures achieved in a muffle furnace? Discover the Science Behind Precision Heating
- Why is a Muffle Furnace required for lithium recovery? Boost Yield with Precise Sulfuric Acid Roasting



















