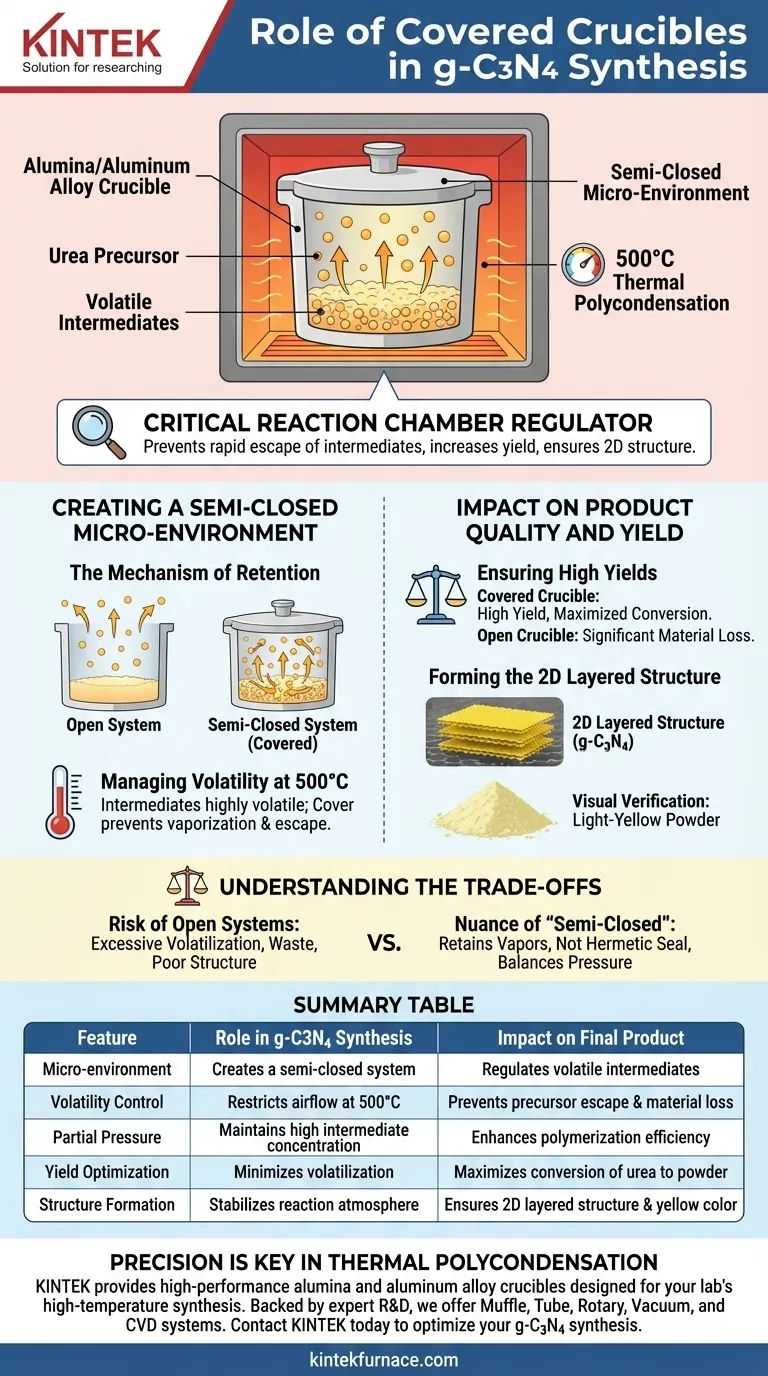
A covered alumina or aluminum alloy crucible acts as a critical reaction chamber regulator. During the thermal polycondensation of urea, the cover creates a semi-closed micro-environment that is essential for managing the behavior of volatile reaction intermediates.
By restricting the airflow, the covered crucible prevents the rapid escape of precursors at high temperatures, directly increasing the yield of graphitic carbon nitride (g-C3N4) and ensuring the formation of its characteristic two-dimensional structure.

Creating a Semi-Closed Micro-Environment
The Mechanism of Retention
The primary function of the cover is to transform an open vessel into a semi-closed system.
When heating urea, the precursor does not simply melt and harden; it undergoes a complex chemical transformation. The cover mechanically restricts the immediate loss of material to the ambient atmosphere.
Managing Volatility at 500°C
The synthesis of g-C3N4 typically requires temperatures around 500°C.
At this temperature, the reaction intermediates generated from urea are highly volatile. Without a physical barrier, these intermediates would vaporize and escape the crucible before they could polymerize into the desired graphitic structure.
Enhancing Reaction Efficiency
The cover maintains a higher partial pressure of the reaction intermediates within the crucible.
This increased concentration forces the intermediates to interact with each other rather than dissipating. This confinement is the key driver for maintaining a high reaction efficiency.
Impact on Product Quality and Yield
Ensuring High Yields
The most immediate benefit of using a covered crucible is a tangible increase in production yield.
By preventing excessive volatilization, a larger percentage of the starting urea is successfully converted into the final product. An open crucible would result in significant material loss.
Forming the 2D Layered Structure
The physical structure of the final material is heavily dependent on the reaction atmosphere.
The semi-closed environment facilitates the proper arrangement of atoms into a two-dimensional layered structure. This structure is what gives g-C3N4 its unique semiconductor properties.
Visual Verification
The success of this process is often visible to the naked eye.
When the semi-closed environment successfully regulates the reaction, the resulting g-C3N4 appears as a light-yellow powder. Deviations in color can often indicate incomplete polymerization or structural defects caused by improper containment.
Understanding the Trade-offs
The Risk of Open Systems
It is a common pitfall to underestimate the volatility of urea intermediates.
Leaving the crucible uncovered promotes excessive volatilization. This not only wastes precursor material but also disrupts the polymerization process, leading to a product with poor structural integrity.
The Nuance of "Semi-Closed"
It is important to note that the system is semi-closed, not hermetically sealed.
The goal is to retain intermediates, not to build dangerous pressure. A simple lid allows for the necessary retention of vapors without creating a pressure vessel, striking the right balance for thermal polycondensation.
Optimizing Your Synthesis Strategy
To ensure you achieve a high-quality g-C3N4 sample, align your equipment choice with your specific goals:
- If your primary focus is Maximizing Yield: You must use a covered crucible to prevent the loss of volatile intermediates at 500°C.
- If your primary focus is Structural Integrity: Rely on the semi-closed environment to facilitate the formation of the correct two-dimensional layered sheets.
Controlling the atmosphere within the crucible is just as critical to the synthesis success as the temperature setting itself.
Summary Table:
| Feature | Role in g-C3N4 Synthesis | Impact on Final Product |
|---|---|---|
| Micro-environment | Creates a semi-closed system | Regulates volatile reaction intermediates |
| Volatility Control | Restricts airflow at 500°C | Prevents precursor escape and material loss |
| Partial Pressure | Maintains high intermediate concentration | Enhances polymerization efficiency |
| Yield Optimization | Minimizes volatilization | Maximizes conversion of urea to powder |
| Structure Formation | Stabilizes reaction atmosphere | Ensures 2D layered structure and yellow color |
Precision is key in thermal polycondensation. KINTEK provides high-performance alumina and aluminum alloy crucibles designed to withstand the rigors of your lab's high-temperature synthesis. Backed by expert R&D and manufacturing, we offer a comprehensive range of lab solutions including Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique research needs. Contact KINTEK today to optimize your g-C3N4 synthesis and ensure superior material quality.
Visual Guide
References
- Chun Zhao, Shaojun Zhang. TiO₂/g-C₃N₄@HPBC Photoanode in PMFC for Shipboard Oily Wastewater Degradation. DOI: 10.54691/kk8pft70
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the key mechanical properties of alumina tubes? Uncover High-Strength, Wear-Resistant Solutions
- What are the advantages of using a laboratory vacuum drying oven for modified ZnO nanomaterials? Protect Nano-Integrity
- What is the function of high-precision molds and laboratory presses in LLTO preparation? Ensure Material Consistency
- How does the gas mixing system in plasma nitriding equipment regulate the quality of the diffusion layer?
- Why is temperature-controlled heating equipment required for calcium perrhenate? Ensure Rhenium Stability at 140 °C
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- Why use high-performance insulation bricks in radiant tube simulations? Ensure precision and industrial accuracy.
- What is the function of the circulating water cooling system? Optimize Pyrolysis Oil Condensation and Yield



















