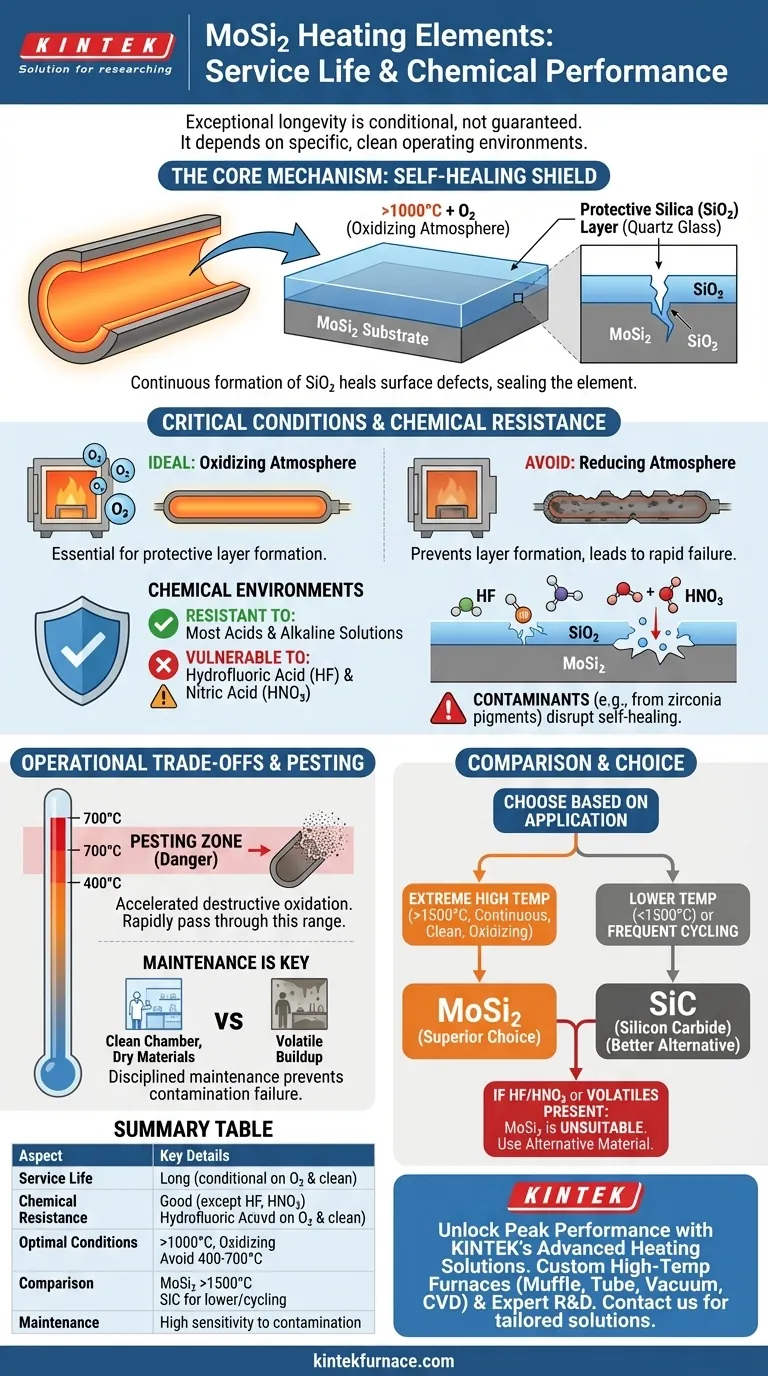
Under the right operating conditions, Molybdenum Disilicide (MoSi₂) heating elements offer an exceptionally long service life due to their unique self-healing properties at high temperatures. While they are highly resistant to most common acidic and alkaline solutions, they are quickly degraded by hydrofluoric acid and nitric acid. The longevity of these elements is not guaranteed; it is directly dependent on maintaining a specific, clean operating environment.
The exceptional service life of a MoSi₂ element is not an inherent property but a conditional outcome. It hinges on the continuous formation of a protective silica layer in an oxygen-rich atmosphere, a process that is easily disrupted by specific chemical contaminants and improper operational procedures.

The Core Mechanism: How MoSi₂ Achieves Longevity
The long life of MoSi₂ elements is not due to the material being inert, but rather due to a controlled, beneficial reaction with its environment at high temperatures.
The Protective Silica (SiO₂) Layer
At temperatures above 1000°C, the surface of the MoSi₂ element reacts with oxygen in the atmosphere. This reaction forms a thin, dense, and non-porous layer of quartz glass (silica, SiO₂).
This silica layer acts as a self-healing shield. If a crack or defect forms on the surface, the exposed MoSi₂ underneath immediately oxidizes, effectively "healing" the breach and restoring the protective barrier.
The Critical Role of an Oxidizing Atmosphere
This self-healing mechanism is entirely dependent on the presence of oxygen. For this reason, MoSi₂ elements are ideally suited for continuous work in furnaces with an oxygen-bearing atmosphere.
Operating in a reducing (oxygen-poor) atmosphere prevents the formation of this protective layer, leading to rapid degradation and significantly shorter service life.
Performance in Chemical Environments
While robust, MoSi₂ elements have very specific chemical weaknesses that can lead to catastrophic failure.
General Chemical Resistance
The stable silica layer that forms on the element's surface is chemically resilient. It does not dissolve in most common acids or alkaline solutions, making it suitable for a wide range of high-temperature processes.
Specific Vulnerabilities: Hydrofluoric and Nitric Acid
Certain chemicals will actively destroy the protective silica layer. Hydrofluoric acid (HF) is particularly destructive, as it readily dissolves silica.
Nitric acid (HNO₃) will also attack the element, leading to rapid failure. The presence of either of these substances, even in vapor form, makes MoSi₂ an unsuitable choice.
The Impact of Process Contaminants
Other contaminants can disrupt the integrity of the silica layer, compromising the element's lifespan. This is a common issue in applications like dental furnaces.
For example, volatile compounds from pigments or glazes used on zirconia can deposit on the element surface. These deposits interfere with the self-healing process, creating weak points that can lead to premature failure.
Understanding the Trade-offs and Limitations
The high-performance nature of MoSi₂ elements comes with specific operational requirements and potential failure modes that must be managed.
The "Pesting" Phenomenon
At intermediate temperatures, typically between 400°C and 700°C, MoSi₂ can undergo a phenomenon known as "pesting." This is a form of accelerated, destructive oxidation that turns the element into powder.
This makes MoSi₂ a poor choice for applications that dwell for long periods in this specific temperature range. They are designed to be heated through this zone relatively quickly.
Sensitivity to Maintenance and Contamination
The references to contamination from painted zirconia highlight a key operational reality: MoSi₂ furnaces require disciplined maintenance.
Technicians must ensure that materials being heated are properly dried and that the furnace chamber is kept clean to prevent the buildup of volatile contaminants that compromise the heating elements.
The SiC Comparison: A Matter of Temperature
MoSi₂ elements generally last longer than Silicon Carbide (SiC) elements when operated continuously above 1500°C.
Below this temperature, or in applications with frequent thermal cycling, the advantages of MoSi₂ are less pronounced, and SiC may offer a more robust or cost-effective solution.
Making the Right Choice for Your Application
To maximize service life, you must match the element's characteristics to your specific process environment and operational discipline.
- If your primary focus is extreme high-temperature (1600°C+) continuous operation: MoSi₂ is the superior choice, provided the atmosphere is clean and consistently oxygen-rich.
- If your process involves hydrofluoric acid, nitric acid, or other volatile contaminants: MoSi₂ elements are unsuitable and will fail prematurely; an alternative material is required.
- If your application operates primarily below 1500°C or involves frequent cycling: Carefully evaluate if Silicon Carbide (SiC) might offer better overall cost-performance and durability.
- If you prioritize operational forgiveness for maintenance staff: The high sensitivity of MoSi₂ to contamination requires a greater level of procedural discipline than some alternative heating elements.
Understanding these operational principles is the key to unlocking the exceptional performance and lifespan of MoSi₂ heating elements.
Summary Table:
| Aspect | Key Details |
|---|---|
| Service Life | Exceptionally long with self-healing in oxygen-rich, clean environments; depends on operating conditions |
| Chemical Resistance | Resistant to most acids and alkalis; vulnerable to hydrofluoric acid (HF) and nitric acid (HNO₃) |
| Optimal Conditions | Operate above 1000°C in oxidizing atmospheres; avoid 400-700°C range to prevent pesting |
| Comparison with SiC | Superior above 1500°C; SiC may be better for lower temperatures or frequent cycling |
| Maintenance Needs | Requires clean environment to prevent contamination from volatiles like pigments or glazes |
Unlock Peak Performance for Your Laboratory with KINTEK's Advanced Heating Solutions
Are you dealing with high-temperature processes that demand reliable and durable heating elements? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions tailored to your needs. Our product line includes Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to precisely meet your unique experimental requirements.
Whether you're optimizing for longevity in oxidizing environments or navigating chemical sensitivities, our expertise ensures you get the right solution for applications like materials testing, research, and industrial heating. Don't let element failures slow you down—contact us today to discuss how we can enhance your lab's efficiency and reliability.
Get in touch now for a customized consultation!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism
- What makes SIC heating elements superior for high-temperature applications? Unlock Efficiency and Durability
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability



















