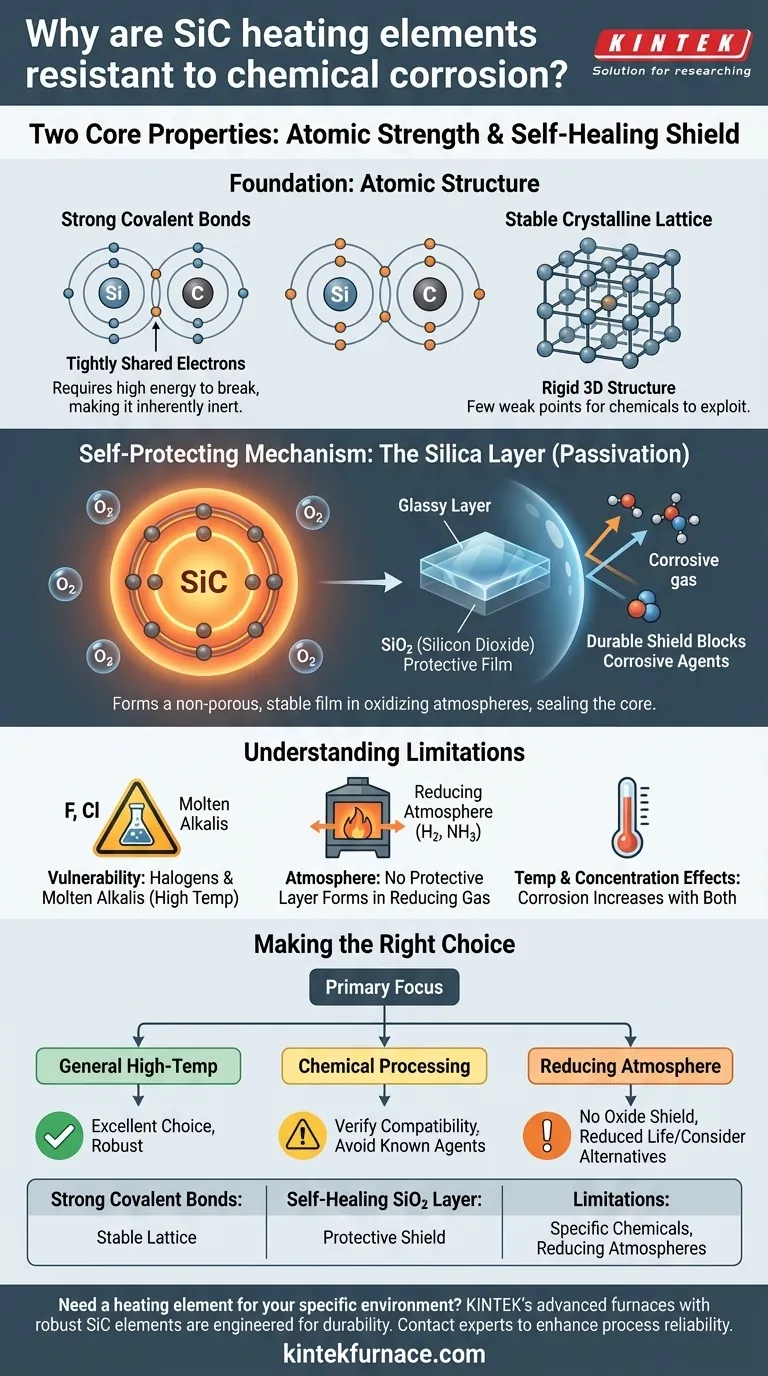
At its core, the chemical resistance of Silicon Carbide (SiC) heating elements stems from two fundamental properties: the immense strength of the atomic bonds between silicon and carbon, and the element's ability to form a stable, self-healing protective layer of silicon dioxide (SiO₂) on its surface when heated. This combination makes the material inherently inert and exceptionally durable in many harsh environments.
The key takeaway is not just that Silicon Carbide is resistant, but how it protects itself. Its strength comes from a passive, glass-like film that forms on its surface, acting as a shield against chemical attack.

The Foundation of Resistance: Atomic Structure
The exceptional properties of Silicon Carbide begin at the atomic level. The way its atoms are bonded together creates a structure that is inherently difficult to break down through chemical reactions.
The Strength of Covalent Bonds
Silicon and carbon atoms in a SiC crystal are linked by powerful covalent bonds. These bonds involve the sharing of electrons, creating an extremely stable and tightly-bound molecular structure.
Breaking these bonds requires a significant amount of energy. This high energy requirement is why SiC does not readily react with most chemicals, making it chemically inert by nature.
A Stable Crystalline Lattice
These covalent bonds form a rigid, three-dimensional crystalline lattice. This structure is not only responsible for SiC's renowned hardness and mechanical strength but also contributes directly to its chemical stability.
There are few "weak points" in the lattice for corrosive chemicals to exploit and initiate a reaction, unlike in materials with weaker metallic or ionic bonds.
The Self-Protecting Mechanism: The Silica Layer
While its atomic structure provides a strong defense, SiC's most dynamic protective feature is a thin film that forms on its surface. This process is known as passivation.
Formation of the Passive Oxide Film
When SiC heating elements are operated in an atmosphere containing oxygen, the surface silicon reacts to form a thin, non-porous layer of silicon dioxide (SiO₂), which is essentially a form of quartz or glass.
This SiO₂ layer is extremely stable and acts as a physical barrier. It effectively seals the underlying Silicon Carbide from direct contact with corrosive gases or liquids in the environment.
Why This Layer is So Effective
The protective SiO₂ film is itself highly resistant to a wide range of acids and other chemicals. It functions as a durable shield that prevents corrosive agents from reaching and degrading the core heating element.
This "self-passivating" behavior is what allows SiC elements to maintain their structural integrity and performance over long periods in aggressive industrial processes.
Understanding the Trade-offs and Limitations
No material is completely immune to all forms of chemical attack. Understanding the specific limitations of Silicon Carbide is critical for proper application and to avoid premature failure.
Vulnerability to Specific Chemicals
SiC's resistance is not universal. It can be attacked by halogens (like fluorine and chlorine) at high temperatures, as well as by molten alkalis (like sodium hydroxide) and certain molten metals.
Always verify the compatibility of SiC with the specific chemical agents present in your furnace atmosphere, especially in less common or highly reactive processes.
The Critical Role of Atmosphere
The protective SiO₂ layer only forms and remains stable in an oxidizing atmosphere. In a strongly reducing atmosphere (such as pure hydrogen or cracked ammonia), this protective layer can be stripped away.
Operating without the SiO₂ shield leaves the SiC material vulnerable to direct chemical attack and can significantly shorten the element's lifespan.
Temperature and Concentration Effects
The rate of corrosion, even for resistant materials, generally increases with temperature and the concentration of the corrosive agent. While SiC performs exceptionally well, its limits must be respected in extreme high-temperature chemical environments.
Making the Right Choice for Your Application
Selecting the right heating element requires matching the material's properties to your specific operational environment. Silicon Carbide's unique profile makes it ideal for certain conditions but requires careful consideration in others.
- If your primary focus is general high-temperature use: SiC is an excellent and robust choice for most standard air or inert gas atmospheres due to its strength and self-protecting nature.
- If your primary focus is chemical processing: Verify that your process chemicals are not among the known agents that attack SiC, such as halogens or molten alkalis at high temperatures.
- If your primary focus is operating in a reducing atmosphere: Be aware that the protective oxide layer may not form, and you may need to consider alternative materials or accept a potentially reduced element life.
By understanding both the inherent strengths and the specific vulnerabilities of Silicon Carbide, you can make an informed decision that ensures reliability and longevity for your process.
Summary Table:
| Key Factor | How It Contributes to Chemical Resistance |
|---|---|
| Strong Covalent Bonds | Creates a stable, inert atomic lattice that is difficult for chemicals to break down. |
| Self-Healing SiO₂ Layer | Forms a protective glass-like shield on the surface when heated in oxygen, sealing the core material. |
| Limitations | Vulnerable to halogens, molten alkalis, and may not form a protective layer in strong reducing atmospheres. |
Need a heating element that can withstand your specific chemical environment?
KINTEK's advanced high-temperature furnaces, equipped with robust SiC heating elements, are engineered for durability in demanding applications. Leveraging our exceptional R&D and in-house manufacturing, we provide diverse laboratories with solutions like Muffle, Tube, and Vacuum Furnaces. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements, including challenging chemical atmospheres.
Contact our experts today to discuss how our furnace solutions can enhance the reliability and longevity of your process.
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Dental Porcelain Zirconia Sintering Ceramic Vacuum Press Furnace
People Also Ask
- What are the properties and applications of silicon carbide (SiC)? Unlock High-Temperature Performance
- Why is silicon carbide resistant to chemical reactions in industrial furnaces? Unlock Durable High-Temp Solutions
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- Why are silicon carbide heating elements essential in high-temperature industries? Unlock Reliable, Extreme Heat Solutions

















