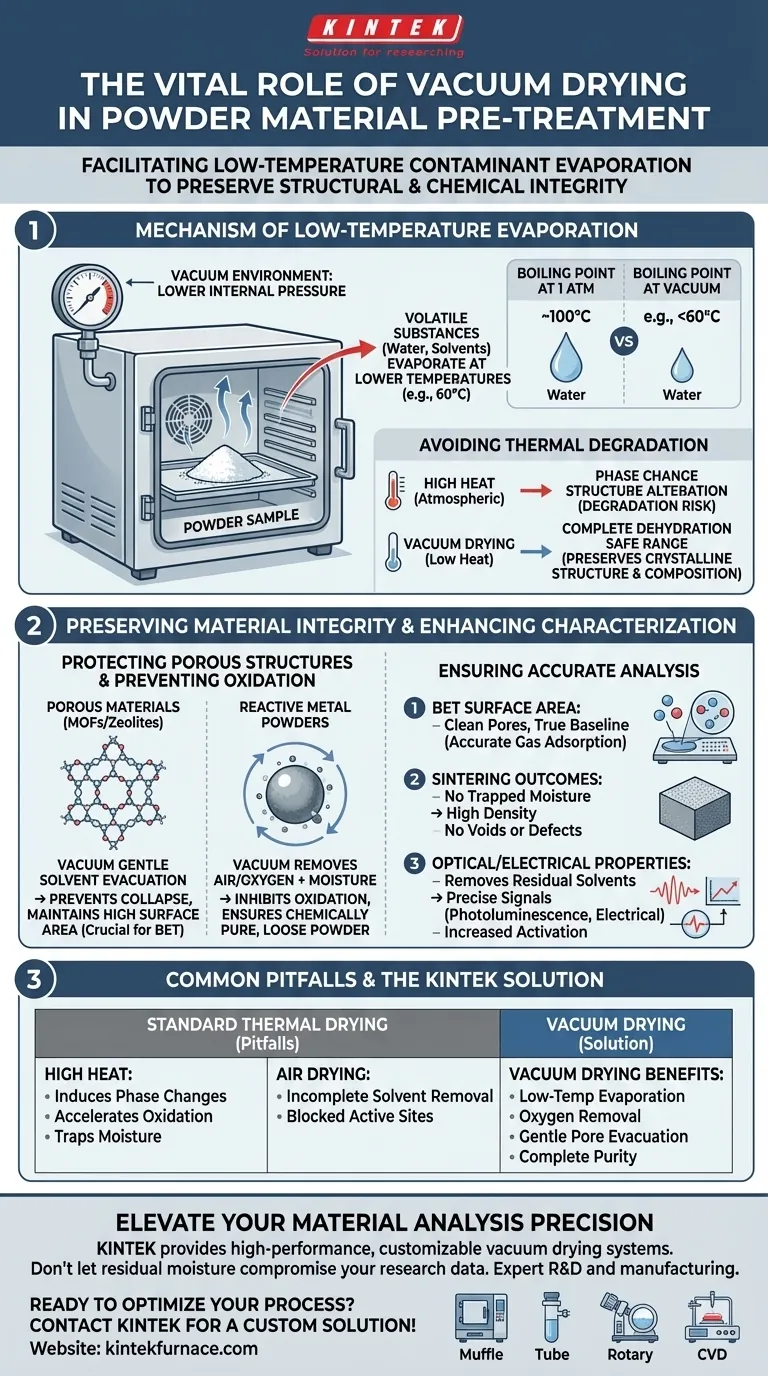
The primary purpose of using a vacuum drying oven is to facilitate the evaporation of moisture, solvents, and organic residues from powder materials at significantly reduced temperatures. By lowering the internal pressure of the chamber, the boiling point of these contaminants drops, allowing for their thorough removal without subjecting the sample to destructive high heat. This process is critical for preserving the structural and chemical integrity of sensitive materials prior to characterization.
Core Insight: Vacuum drying is a preservation strategy, not just a drying method. Its main value lies in decoupling evaporation from high thermal energy, ensuring that sensitive microstructures (like pores in MOFs) remain intact and reactive surfaces remain unoxidized for accurate downstream analysis.

The Mechanism of Low-Temperature Evaporation
Lowering the Boiling Point
The fundamental advantage of this equipment is the manipulation of thermodynamics.
By creating a vacuum environment, the system reduces the pressure surrounding the material. This allows volatile substances, such as water or washing solvents like ethanol, to boil and evaporate at temperatures far below their standard boiling points (e.g., drying at 60°C).
Avoiding Thermal Degradation
Many advanced materials undergo phase changes or degradation when exposed to the high temperatures required for drying at atmospheric pressure.
Vacuum drying bypasses this risk. It allows for complete dehydration while keeping the processing temperature within a safe range, preventing the material from altering its crystalline structure or chemical composition.
Preserving Material Integrity
Protecting Porous Structures (MOFs and Zeolites)
Materials with complex internal geometries, such as Metal-Organic Frameworks (MOFs) and zeolites, are highly susceptible to structural collapse.
If these materials are heated excessively to remove trapped solvents, their internal framework may disintegrate. Vacuum drying gently removes physically adsorbed molecules from the pores, preventing blockage and collapse, which is essential for maintaining the material's high surface area.
Preventing Oxidation in Metal Powders
For reactive materials like metal powders, the presence of oxygen and high heat creates a perfect environment for oxidation.
Vacuum drying removes both moisture and air (oxygen) simultaneously. This prevents the formation of oxide layers on the particle surface, which ensures the powder remains chemically pure and loose for subsequent processing steps like grinding or sintering.
Enhancing Characterization Accuracy
Ensuring Accurate Surface Area Analysis (BET)
Techniques like BET surface area testing rely on measuring gas adsorption into open pores.
If residual moisture or organics remain in these pores, the resulting data will be skewed. Vacuum pre-treatment ensures the pores are completely empty and the surface is "clean," providing a true baseline for surface area measurements.
Improving Sintering Outcomes
In powder metallurgy, trapped moisture can be disastrous during the sintering phase.
If moisture is not removed beforehand, it will evaporate rapidly during high-temperature sintering, creating voids, pores, or oxidation defects in the final bulk material. Vacuum drying prevents this, ensuring high density in the final sintered product.
Optimizing Optical and Electrical Measurements
For nano-materials, residual solvents can interfere with photoluminescence and electrical property signals.
By thoroughly evacuating solvent molecules, vacuum drying increases the material's degree of activation. This clarity is required to obtain precise, noise-free data regarding the material's optical and electrical performance.
Common Pitfalls to Avoid
The Risk of Standard Thermal Drying
A common error is assuming that a standard laboratory oven is sufficient for all powder drying.
Standard ovens rely on heat alone to drive off moisture. For sensitive composites (like Bi2SiO5), this heat can induce unwanted phase changes. For metals, it accelerates oxidation. Standard drying often traps moisture deep within pores, leading to data errors that are difficult to trace later.
Incomplete Solvent Removal
Simply air-drying powders often leaves "bound" solvents trapped in micro-pores.
Without the negative pressure of a vacuum, these solvents may not possess the energy to escape deep internal structures. This residual solvent can act as a contaminant, effectively blocking the active sites of the material and rendering characterization tests inaccurate.
Making the Right Choice for Your Goal
To ensure your characterization data is reliable, align your pre-treatment with your specific analytical goals:
- If your primary focus is Surface Area (BET): Use vacuum drying to gently evacuate physically adsorbed molecules without collapsing the delicate pore structure.
- If your primary focus is Sintering/Density: Use vacuum drying to prevent oxidation defects and void formation caused by moisture expansion at high heat.
- If your primary focus is Optical/Electrical Properties: Use vacuum drying to fully remove washing solvents (like ethanol) that would otherwise dampen signals or block active sites.
Effective pre-treatment is the invisible variable that determines the reproducibility and accuracy of your final data.
Summary Table:
| Feature | Vacuum Drying Benefit | Impact on Characterization |
|---|---|---|
| Temperature Control | Low-temperature evaporation | Prevents thermal degradation and phase changes |
| Atmospheric Control | Removal of air/oxygen | Inhibits oxidation of reactive metal powders |
| Pore Preservation | Gentle solvent evacuation | Maintains internal structures (MOFs/Zeolites) for BET |
| Purity | Complete moisture removal | Eliminates voids and defects during sintering/analysis |
Elevate Your Material Analysis Precision
Don't let residual moisture or thermal damage compromise your research data. KINTEK provides high-performance, customizable vacuum drying systems designed to protect your sensitive powders and ensure reproducible results. Backed by expert R&D and manufacturing, we offer a comprehensive range of lab high-temp equipment, including Muffle, Tube, Rotary, Vacuum, and CVD systems, tailored to your unique specifications.
Ready to optimize your pre-treatment process? Contact KINTEK today for a custom solution!
Visual Guide
References
- Jianjun Ma, Qiuhong Zhou. Galvanic Displacement Engineered Pt/Co₃O₄‐CeO₂ for High‐Efficiency Toluene Elimination at Low Temperature. DOI: 10.1002/slct.202405496
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Which industries heavily rely on graphite in vacuum furnaces? Powering High-Performance Manufacturing
- What types of heat treatment processes can be performed in a vacuum furnace? Unlock Superior Material Quality and Control
- What are the stages of the heat treatment process in drop-bottom quench furnaces? Achieve Superior Hardness and Strength
- Why is a Vacuum Oven utilized for the final drying of BC-Fe3O4 nanoparticles? Preserve Purity and Porosity
- Which industries benefit from vacuum furnaces? Unlock Material Perfection for Aerospace, Medical, and More
- What energy-saving and environmental benefits do vacuum sintering furnaces offer? Boost Efficiency and Cut Emissions
- What are the critical steps in the vacuum arc furnace process? Achieve Unmatched Metal Purity and Performance
- How does increasing the vacuum furnace annealing temperature to 900 K adversely affect Ti–TEG composites?



















