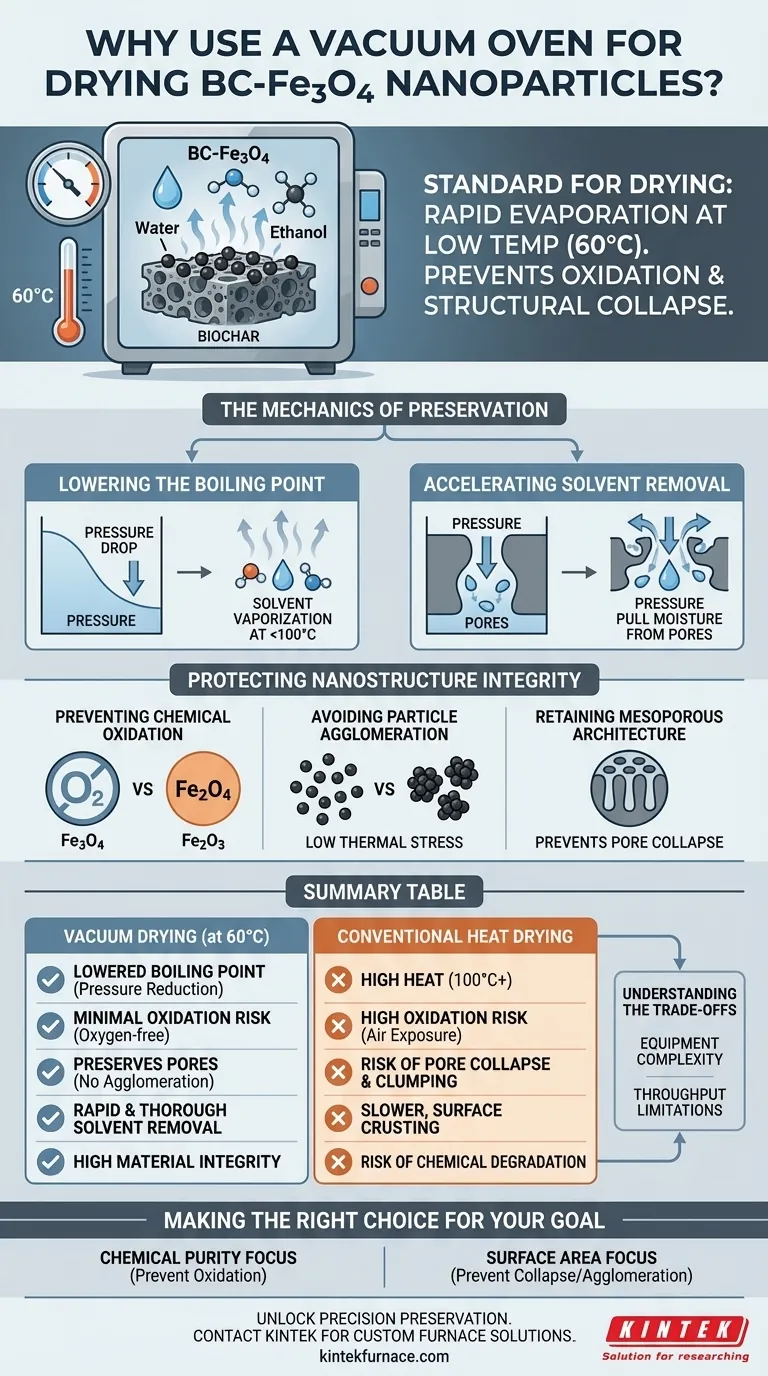
A vacuum oven is the standard for drying BC-Fe3O4 nanoparticles because it enables the rapid evaporation of solvents like water and ethanol at a low temperature, specifically around 60°C. By lowering the atmospheric pressure, this technique protects the material from the oxidation and structural collapse that often occur with conventional high-heat drying methods.
By decoupling temperature from evaporation speed, vacuum drying preserves the delicate mesoporous structure and surface chemistry of the biochar composite, preventing the iron oxide from degrading into less active forms.

The Mechanics of Preservation
Lowering the Boiling Point
The primary mechanism at work is the relationship between pressure and boiling points. By creating a negative pressure environment, the vacuum oven allows residual solvents to vaporize rapidly without requiring the material to reach 100°C or higher.
Accelerating Solvent Removal
This method is particularly effective for removing stubborn solvents trapped within the porous structure. The pressure differential actively pulls moisture and ethanol out of the material, ensuring a thorough dry at a safe, controlled temperature of 60°C.
Protecting Nanostructure Integrity
Preventing Chemical Oxidation
Iron oxides, such as Fe3O4, are highly susceptible to oxidation when heated in the presence of air. A vacuum environment removes oxygen from the chamber, ensuring the iron remains in its active state and does not degrade into less useful oxides like rust (hematite).
Avoiding Particle Agglomeration
High thermal energy often causes nanoparticles to clump together, or agglomerate, which drastically reduces their surface area. Low-temperature vacuum drying minimizes this thermal stress, keeping the nanoparticles distinct and well-distributed across the biochar support.
Retaining Mesoporous Architecture
Biochar (BC) relies on its porous structure for performance. Vacuum drying prevents the collapse of these pores (morphology collapse) and preserves the surface functional groups, which are critical for the material's chemical reactivity and adsorption capabilities.
Understanding the Trade-offs
Equipment Complexity
Unlike standard convection ovens, vacuum drying requires a vacuum pump and a sealed chamber. This adds layers of mechanical complexity and requires regular maintenance of seals and oil to ensure a consistent vacuum is held.
Throughput Limitations
Vacuum drying is generally a batch process with limited capacity compared to continuous belt dryers. It is ideal for high-value precision materials like BC-Fe3O4 but may become a bottleneck if scaling up to massive industrial quantities without specialized equipment.
Making the Right Choice for Your Goal
To ensure you are optimizing your synthesis process, consider your specific performance targets:
- If your primary focus is Chemical Purity: Rely on vacuum drying to strictly prevent the oxidation of Fe3O4 to Fe2O3, ensuring your magnetic and catalytic properties remain intact.
- If your primary focus is Surface Area: Use this method to prevent pore collapse and agglomeration, maximizing the accessible surface area for adsorption applications.
The vacuum oven is not just a drying tool; it is a preservation chamber that locks in the chemical and structural potential of your nanocomposite.
Summary Table:
| Feature | Vacuum Drying (at 60°C) | Conventional Heat Drying |
|---|---|---|
| Boiling Point | Lowered via pressure reduction | Requires high heat (100°C+) |
| Oxidation Risk | Minimal (Oxygen-free environment) | High (Exposure to air and heat) |
| Particle Structure | Preserves pores; no agglomeration | Risk of pore collapse and clumping |
| Solvent Removal | Rapid and thorough extraction | Slower; surface crusting possible |
| Material Integrity | High preservation of biochar | Risk of chemical degradation |
Unlock Precision Preservation for Your Nanocomposites
Maintaining the structural integrity of materials like BC-Fe3O4 requires more than just heat—it requires a controlled environment. KINTEK provides industry-leading vacuum drying solutions designed to protect your most sensitive lab-scale and industrial materials.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all of which are fully customizable to meet your unique research or production needs. Whether you are aiming to prevent oxidation or maximize surface area, our high-temp furnace technology ensures your nanoparticles remain in their most active state.
Ready to elevate your synthesis results? Contact KINTEK today to discuss your custom furnace solution.
Visual Guide
References
- Meenakshi Sundaram Sharmila, Gurusamy, Annadurai. Biogenic fabrication of biochar-functionalized iron oxide nanoparticles using Miscanthus sinensis for oxytetracycline removal and toxicological assessment. DOI: 10.12692/jbes/27.2.10-20
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the function of introducing 150 Pa of argon gas into a furnace during the vacuum refining of AM60 magnesium alloy?
- Why is it necessary to use a vacuum drying oven for Silicon Carbide slurry? Enhance Purity and Green Body Density
- How are active connection parts in a vacuum furnace sealed? Discover the Role of O-Rings and Water Cooling
- Why is a high vacuum furnace necessary for the solution treatment of cold-rolled TNZTSF alloys? Prevent Oxidation.
- Why is a vacuum drying oven required for Se/PPS composite treatment at 110°C? Ensure Chemical Purity & Bond Strength
- How does vacuum sintering improve surface finish? Achieve Superior, Oxide-Free Results
- Why is a 'baking-out' pretreatment necessary for magnesium purification? Ensure Ultra-High Purity in Your Vacuum Distillation
- What are the specific requirements for the drying process in a vacuum drying oven? Essential MXene-ZrB2 Prep Steps



















