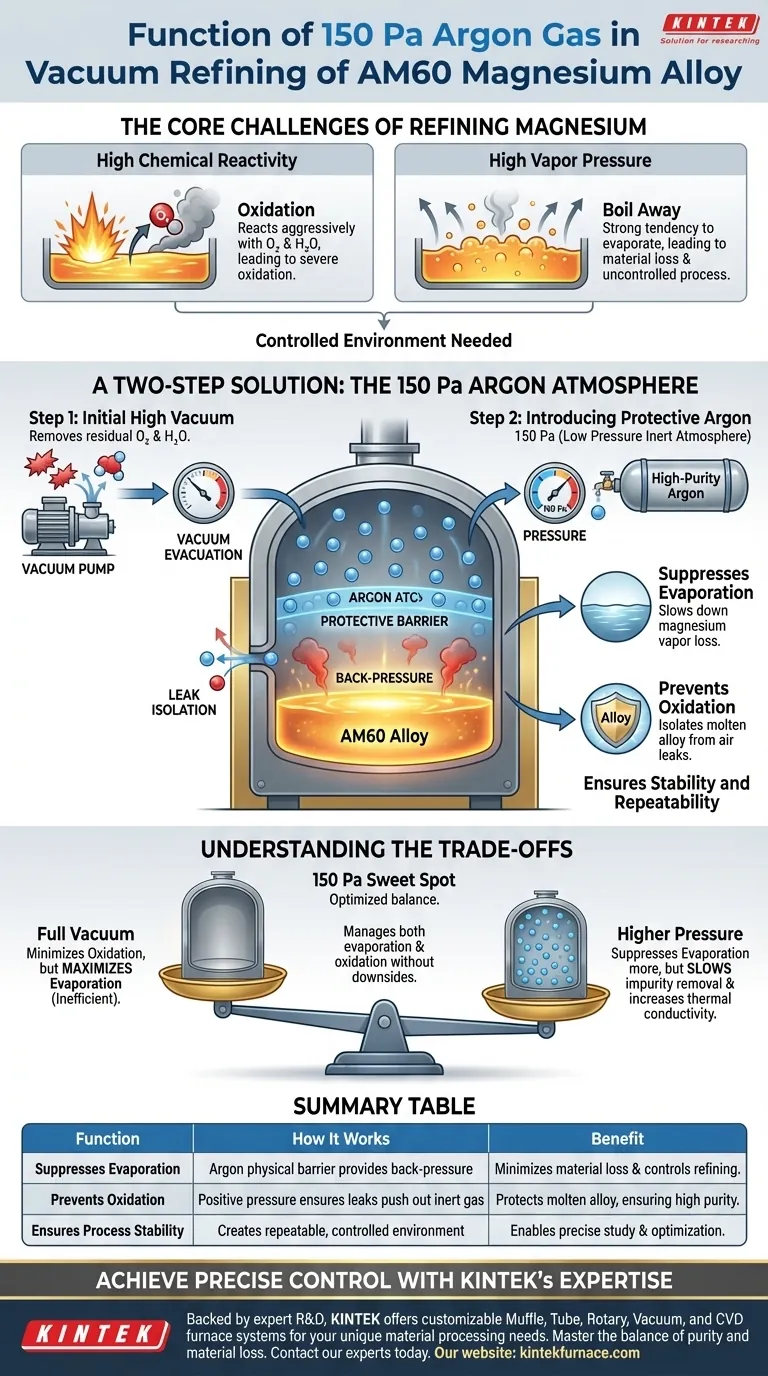
In the vacuum refining of AM60 magnesium alloy, introducing 150 Pa of argon gas is a critical control measure. This action establishes a low-pressure, inert atmosphere that serves two primary functions: it physically suppresses the rapid evaporation of magnesium vapor from the melt, and it provides a protective barrier that isolates the molten alloy from any potential air leaks, thereby preventing oxidation.
The core challenge in refining magnesium isn't just preventing oxidation, but also controlling its tendency to boil away at high temperatures. A 150 Pa argon atmosphere creates the precise "sweet spot" of pressure needed to manage this evaporation without reintroducing other contaminants.

The Core Challenges of Refining Magnesium
To understand the function of the argon atmosphere, we must first appreciate the two fundamental properties that make magnesium challenging to work with at high temperatures.
High Chemical Reactivity
Magnesium is an extremely reactive metal. When heated to a molten state, it will readily and aggressively react with any oxygen or water vapor present, leading to severe oxidation and significant loss of material.
High Vapor Pressure
Independent of oxidation, magnesium also has a high vapor pressure. This means it has a strong natural tendency to evaporate, or "boil," turning from a liquid into a gas at the temperatures required for refining. This leads to material loss and makes the process difficult to control.
A Two-Step Solution for a Controlled Environment
The refining process uses a precise, two-step atmospheric control method to counteract both of these challenges.
Step 1: Initial High Vacuum
Before heating begins, the furnace chamber is evacuated to a high vacuum. This initial step is critical for removing as much residual air—specifically oxygen and water vapor—as possible from the system. This creates a clean, inert environment that minimizes the risk of oxidation from the start.
Step 2: Introducing the Protective Argon Atmosphere
Once evacuated, the furnace is backfilled with a small amount of high-purity argon gas to a stable pressure of 150 Pa. This low-pressure atmosphere performs two specific and crucial jobs simultaneously.
Suppressing Evaporation
The argon atoms create a physical barrier over the surface of the molten magnesium. This layer provides just enough "back-pressure" to significantly slow down the rate at which magnesium atoms can escape the liquid and turn into vapor, suppressing the rapid and uncontrolled evaporation that would occur in a pure vacuum.
Preventing Oxidation
Because the furnace contains a positive pressure of argon, any minor leaks in the system will cause argon to leak out, rather than allowing ambient air to leak in. This effectively isolates the highly reactive molten magnesium from any external oxygen, providing a robust defense against oxidation throughout the process.
Ensuring Stability and Repeatability
This precisely controlled atmosphere creates stable and repeatable conditions. By managing both oxidation and evaporation, operators can precisely study and control the refining process, ensuring consistent results.
Understanding the Trade-offs
The choice of 150 Pa of argon over a full vacuum or ambient pressure is a deliberate engineering compromise.
Why Not a Full Vacuum?
While a hard vacuum would be superior for preventing oxidation, it offers zero resistance to evaporation. This would maximize the uncontrolled loss of magnesium vapor, making it an inefficient and impractical choice.
Why Not a Higher Pressure?
Using a significantly higher pressure of argon would suppress evaporation even more, but it would also introduce downsides. It would slow down the removal of other volatile impurities from the melt and increase thermal conductivity, potentially altering the energy requirements of the furnace. The 150 Pa level is the optimized balance point.
How to Apply This to Your Process
Your specific operational goal will determine which aspect of this process is most critical to monitor.
- If your primary focus is maximizing purity: The thoroughness of the initial high-vacuum evacuation is your most critical step to eliminate reactive gases like oxygen.
- If your primary focus is minimizing material loss: The stability and precision of the 150 Pa argon atmosphere is essential for controlling magnesium evaporation.
- If your primary focus is process efficiency: Using argon for both refining (control) and post-process cooldown (to accelerate cooling and prevent re-oxidation) is key to shortening cycle times.
Ultimately, the precise use of an argon atmosphere transforms the refining process from a fight against magnesium's natural volatility into a highly controlled and repeatable operation.
Summary Table:
| Function | How It Works | Benefit |
|---|---|---|
| Suppresses Evaporation | Argon atoms create a physical barrier, providing back-pressure to slow magnesium vapor loss. | Minimizes material loss and controls the refining process. |
| Prevents Oxidation | Positive argon pressure ensures any leaks push inert gas out, preventing air (oxygen) from entering. | Protects the molten alloy, ensuring high purity. |
| Ensures Process Stability | Creates a repeatable, controlled environment for consistent results. | Enables precise study and optimization of the refining cycle. |
Achieve precise control over your high-temperature processes.
Refining reactive metals like magnesium requires exact atmospheric control to balance purity and material loss. The detailed explanation above shows how critical a stable, inert environment is for success.
KINTEK's expertise can help you master this balance. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD furnace systems, all customizable for your unique material processing needs. Whether you are working with alloys, ceramics, or other advanced materials, our lab high-temperature furnaces are engineered for reliability and precision.
Ready to enhance your refining process with a furnace designed for optimal atmospheric control? Contact our experts today to discuss your application and discover the perfect solution for your lab.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What does a vacuum furnace do? Achieve Superior Material Processing in a Pure Environment
- What is the heat treatment in a vacuum furnace? Achieve Superior Metallurgical Properties
- How does vacuum heat treatment improve mechanical properties of metals? Enhance Strength and Durability
- What role does a high-temperature vacuum heat treatment furnace play in TBC post-processing? Enhance Coating Adhesion
- What is a vacuum furnace used for? Achieve Purity and Precision in High-Temp Processing



















