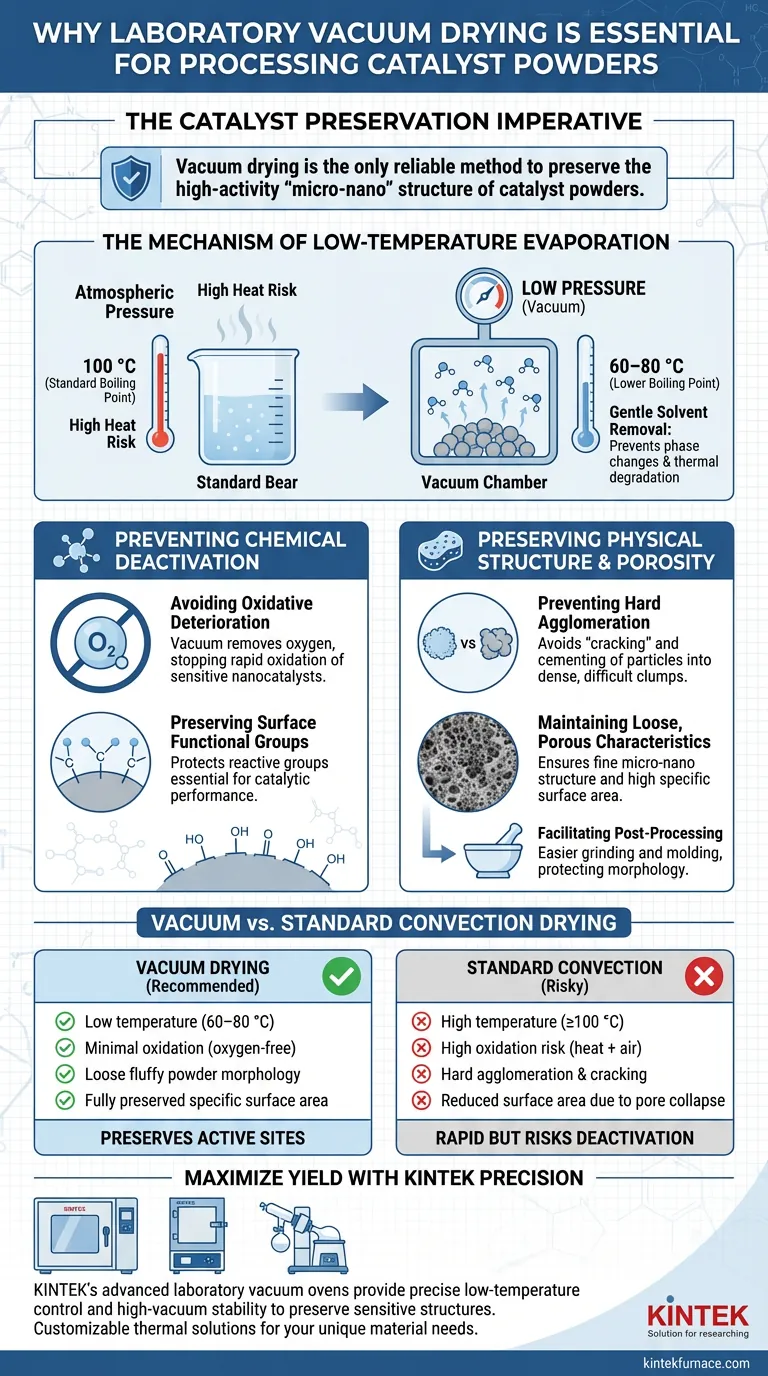
The necessity of using a laboratory vacuum drying oven lies in its ability to decouple evaporation from high heat. By creating a negative pressure environment, these ovens allow moisture and solvents to evaporate at significantly lower temperatures (often 60–80 °C), protecting the catalyst from the thermal and oxidative damage inherent in standard drying methods.
Core Takeaway Vacuum drying is the only reliable method to preserve the high-activity "micro-nano" structure of catalyst powders. It prevents the irreversible loss of active sites caused by oxidative deterioration and the physical destruction of porosity caused by hard agglomeration.

The Mechanism of Low-Temperature Evaporation
Lowering the Boiling Point
The primary function of the vacuum oven is to lower the ambient pressure surrounding the sample. This physical change significantly reduces the boiling point of water and organic solvents like ethanol.
Gentle Solvent Removal
Because the boiling point is lowered, solvents can be removed rapidly without subjecting the material to extreme heat. This is critical for preventing phase changes or thermal degradation that often occur at standard boiling temperatures (e.g., 100 °C for water).
Preventing Chemical Deactivation
Avoiding Oxidative Deterioration
High-activity nanocatalysts are chemically sensitive. Exposing them to a combination of high heat and atmospheric oxygen for prolonged periods leads to rapid oxidative deterioration.
Preserving Surface Functional Groups
The vacuum environment effectively removes oxygen from the chamber. This prevents the premature decomposition or deactivation of surface functional groups (such as nitro groups) that are essential for the catalyst's chemical reactivity.
Preserving Physical Structure and Porosity
Preventing Hard Agglomeration
Standard high-temperature drying often causes "cracking" or cementing of particles as moisture leaves the material. This results in hard agglomeration, creating dense clumps that are difficult to break down.
Maintaining Loose, Porous Characteristics
Vacuum drying prevents these hard structures from forming. It ensures the final powder remains loose and fluffy, which preserves the fine micro-nano structure and the high specific surface area required for optimal catalytic performance.
Facilitating Post-Processing
Because the powder does not agglomerate into hard masses, it is much easier to grind and mold in subsequent steps. This mechanical ease protects the material from the physical stress of intense grinding, which could otherwise destroy the catalyst's morphology.
The Risks of Conventional Drying
Loss of Active Surface Area
If you utilize a standard convection oven, surface tension forces during high-temperature evaporation can collapse the material's pores. This collapse drastically reduces the specific surface area, rendering the catalyst less effective.
Inconsistent Activity
Without vacuum assistance, moisture or air bubbles may remain trapped deep within powder clusters. This residual contamination can lead to unpredictable results during activity evaluation or issues with molding quality later in the process.
Making the Right Choice for Your Goal
While vacuum drying is generally superior for catalysts, understanding your specific objective helps fine-tune the process.
- If your primary focus is Chemical Reactivity: Ensure the vacuum level is sufficient to remove oxygen completely to prevent the oxidation of sensitive elemental nanoparticles (like Platinum or Bismuth).
- If your primary focus is Structural Morphology: Prioritize the low-temperature setting (e.g., 60 °C) to ensure the pore structure does not collapse due to thermal stress.
Ultimately, vacuum drying is not just a drying step; it is a preservation technique essential for maintaining the high-performance potential of synthesized catalysts.
Summary Table:
| Feature | Vacuum Drying Oven | Standard Convection Oven |
|---|---|---|
| Drying Temperature | Low (60–80 °C) | High (≥100 °C) |
| Oxidation Risk | Minimal (Oxygen-free) | High (Heat + Air exposure) |
| Powder Morphology | Loose, fluffy, porous | Hard agglomeration & cracking |
| Specific Surface Area | Fully preserved | Reduced due to pore collapse |
| Core Benefit | Preserves active sites | Rapid but risks deactivation |
Maximize Your Catalyst Yield with KINTEK Precision
Don't let thermal degradation compromise your research. KINTEK’s advanced laboratory vacuum ovens are engineered to provide the precise low-temperature control and high-vacuum stability required to preserve sensitive micro-nano structures.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique material needs. Whether you are processing noble metal nanoparticles or complex porous frameworks, our systems ensure consistent, high-activity results every time.
Ready to upgrade your laboratory drying process? Contact us today to find the perfect thermal solution!
Visual Guide
References
- Chengyu Zhang, Zhisheng Yu. Electronic configuration regulation of single-atomic Mn sites mediated by Mo/Mn clusters for an efficient hydrogen evolution reaction. DOI: 10.1039/d3sc06053e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- How do industrial furnaces and contact voltage regulators facilitate heat transfer performance testing for sodium heat pipes?
- What is the function of a vacuum furnace in phosphor synthesis? Achieve Pure Tb3+/Ce3+ Doped Wollastonite Materials
- What is the role of a vacuum drying oven in N-TiO2@NC preparation? Preserve MXene Integrity and Chemical Stability
- How is vacuum heat treatment applied to elastic alloys? Unlock Peak Performance in Aerospace and Medical Devices
- How do Vacuum Spark Plasma Sintering (SPS) systems compare to traditional furnaces? Achieve Nanometric Grain Control
- What are the advantages of a plasma-assisted electric arc furnace? Boost Your Carbothermic Reduction Efficiency
- What is the function of a vacuum drying oven in rice husk carbonization? Ensure Chemical Stability & Material Integrity
- How do the structural features of a box furnace and a vacuum furnace differ? Compare for Your Lab's Needs



















