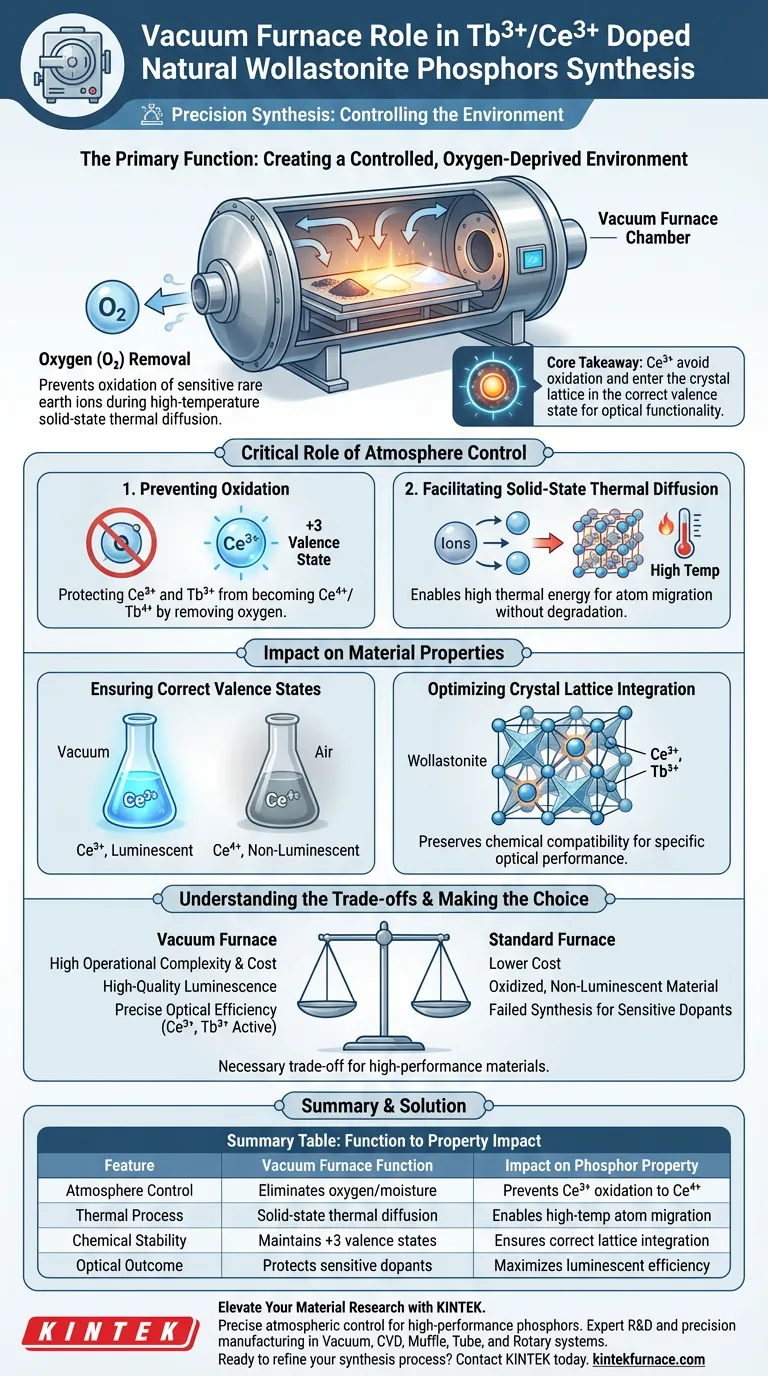
The primary function of a vacuum furnace in the synthesis of Tb3+/Ce3+ doped natural wollastonite phosphors is to create a controlled, oxygen-deprived environment. By maintaining high vacuum levels during the high-temperature solid-state thermal diffusion process, the furnace effectively prevents the oxidation of sensitive rare earth ions.
Core Takeaway Achieving high-performance phosphors requires precise chemical stability at high temperatures. The vacuum furnace ensures that activators, particularly Cerium ($Ce^{3+}$), avoid oxidation and enter the crystal lattice in the correct valence state, which is a prerequisite for the material's optical functionality.

The Critical Role of Atmosphere Control
Preventing Oxidation of Rare Earth Ions
The synthesis process involves heating materials to extreme temperatures, a condition that typically accelerates oxidation.
The vacuum furnace counteracts this by removing oxygen from the reaction chamber. This is specifically required to protect rare earth ions, such as Ce3+, which are highly susceptible to losing electrons and oxidizing into a higher valence state if exposed to air.
Facilitating Solid-State Thermal Diffusion
The synthesis relies on solid-state thermal diffusion, where atoms migrate into the host material structure under heat.
This diffusion process requires high thermal energy to be effective. The vacuum furnace allows the material to reach these necessary temperatures without the chemical degradation that would occur in an oxygen-rich atmosphere.
Impact on Material Properties
Ensuring Correct Valence States
For a phosphor to function, the dopant ions must maintain a specific electronic configuration.
The vacuum environment ensures that the activators remain in their intended +3 valence state (e.g., $Tb^{3+}$ and $Ce^{3+}$). If these ions were to oxidize (for example, $Ce^{3+}$ becoming $Ce^{4+}$), the material would lose its desired luminescent properties.
Optimizing Crystal Lattice Integration
Optical performance is dictated by how well the dopant ions fit into the host's crystal structure.
By preserving the correct valence state, the vacuum furnace ensures the activators are chemically compatible with the natural wollastonite lattice. This precise integration is critical for achieving the specific optical performance and efficiency expected from the phosphor.
Understanding the Trade-offs
Process Complexity vs. Material Quality
Using a vacuum furnace introduces significantly higher operational complexity and equipment costs compared to standard air-atmosphere furnaces.
However, this is a necessary trade-off. Attempting this specific synthesis in a standard furnace would likely result in oxidized, non-luminescent material, rendering the process futile despite the lower cost.
Making the Right Choice for Your Goal
To determine the correct synthesis setup for your project, consider your specific chemical requirements:
- If your primary focus is Optical Efficiency: Prioritize high-vacuum processing to maximize the concentration of active $Ce^{3+}$ and $Tb^{3+}$ ions within the lattice.
- If your primary focus is Cost Reduction: You must verify if alternative, less sensitive dopants can be used, as $Ce^{3+}$ strictly requires a reducing or inert atmosphere.
By controlling the reaction environment, you convert raw potential into precise optical performance.
Summary Table:
| Feature | Vacuum Furnace Function | Impact on Phosphor Property |
|---|---|---|
| Atmosphere Control | Eliminates oxygen/moisture | Prevents $Ce^{3+}$ oxidation to non-luminescent $Ce^{4+}$ |
| Thermal Process | Solid-state thermal diffusion | Enables high-temp atom migration without degradation |
| Chemical Stability | Maintains +3 valence states | Ensures activators integrate correctly into crystal lattice |
| Optical Outcome | Protects sensitive dopants | Maximizes luminescent efficiency and color purity |
Elevate Your Material Research with KINTEK
Precise atmospheric control is the difference between a high-performance phosphor and a failed synthesis. KINTEK provides industry-leading thermal solutions designed to meet the rigorous demands of rare earth processing.
Backed by expert R&D and precision manufacturing, we offer a comprehensive range of Vacuum, CVD, Muffle, Tube, and Rotary systems, all of which are fully customizable to your specific research or production needs. Whether you are optimizing optical efficiency or scaling solid-state diffusion, our high-temperature furnaces deliver the stability and vacuum integrity your project requires.
Ready to refine your synthesis process? Contact KINTEK today to discuss your unique laboratory requirements with our specialists.
Visual Guide
References
- YU Xin-hong, Wei Feng. Anti-thermal-quenching and colour-tuneable Tb3+/Ce3+-doped phosphor from natural wollastonite. DOI: 10.2298/pac2404395y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What are the specific process advantages of using a vacuum drying oven for powder drying? Enhance Material Purity
- How does a gas pressure furnace facilitate the densification of Si3N4–SiC–MoSi2? Achieving Near-Theoretical Density
- How do custom vacuum furnaces ensure precise temperature control? Achieve Superior Thermal Uniformity for Your Lab
- What are the key applications of laboratory vacuum furnaces? Unlock High-Purity Material Processing
- What are the size ranges and common applications of crucible furnaces? Find Your Perfect Fit for Small-Batch Melting
- What advantages does the non-linear processing in a vacuum furnace offer? Achieve Precise Material Control
- How is the vacuum created in a vacuum furnace? Master the Process for Superior Metallurgical Results
- What types of materials are processed using vacuum resistance furnaces? Ideal for Reactive Metals, Alloys, and Ceramics



















