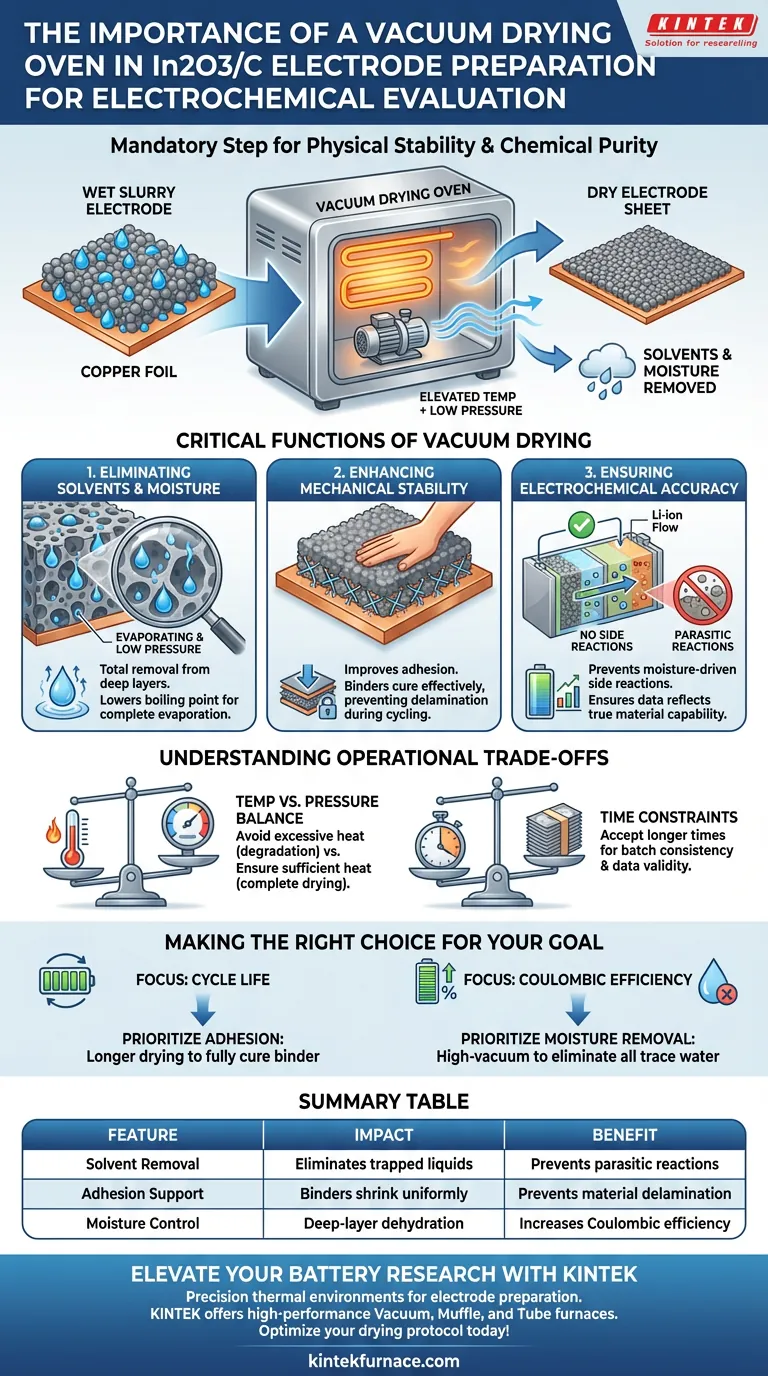
The use of a vacuum drying oven is a mandatory step in preparing In2O3/C negative electrode sheets to ensure the physical stability and chemical purity of the sample. By combining elevated temperatures with a low-pressure environment, this process thoroughly removes slurry solvents and trace moisture that would otherwise compromise the electrode. This is essential for securing the active material to the current collector and preventing contaminants from skewing your electrochemical data.
By lowering the environmental pressure, vacuum drying facilitates the rapid and complete evaporation of volatile components without requiring damagingly high temperatures. This ensures the electrode sheet is physically robust and chemically inert prior to cell assembly.

The Critical Functions of Vacuum Drying
Eliminating Solvents and Moisture
The primary function of the vacuum oven is the total removal of solvents used in the slurry preparation.
Under standard atmospheric pressure, solvents and trace moisture can remain trapped deep within the porous electrode structure.
Creating a vacuum lowers the boiling point of these liquids, forcing them to evaporate completely, even from the deepest layers of the coating.
Enhancing Mechanical Stability
A critical, often overlooked benefit is the improvement of adhesion between the active material and the current collector.
As the solvent evaporates in a controlled vacuum environment, the binder within the In2O3/C composite shrinks and hardens effectively.
This prevents the electrode material from delaminating (peeling off) the copper foil during the cutting process or subsequent battery cycling.
Ensuring Electrochemical Accuracy
Residual water or solvent is a major source of error in electrochemical evaluations.
Moisture trapped in the electrode can react with the electrolyte, leading to parasitic side reactions that consume lithium ions and generate gas.
Vacuum drying prevents these reactions, ensuring that the performance data you collect reflects the true capability of the In2O3/C material, not the interference of contaminants.
Understanding the Operational Trade-offs
Temperature vs. Pressure Balance
While the vacuum allows for drying at lower temperatures, selecting the correct thermal setting is still vital.
As noted in comparative processes, excessive heat can degrade binders or alter the microscopic morphology of the composite.
Conversely, relying solely on vacuum without sufficient heat may result in a "surface dry" condition where moisture remains trapped at the interface with the copper foil.
Time Constraints
Vacuum drying is rarely a fast process; it is a rate-limiting step in electrode fabrication.
Rushing this stage often leads to inconsistency between batches.
You must accept the trade-off of longer preparation times to guarantee the validity of your downstream testing.
Making the Right Choice for Your Goal
To ensure valid results, tailor your drying protocol to your specific evaluation metrics:
- If your primary focus is Cycle Life: Prioritize adhesion. Ensure the drying process is long enough to fully cure the binder, preventing material detachment during repeated charge/discharge cycles.
- If your primary focus is Coulombic Efficiency: Prioritize moisture removal. Use a high-vacuum setting to eliminate every trace of water, as this is the primary cause of irreversible capacity loss and side reactions.
The vacuum drying oven acts as the final gatekeeper of quality, transforming a wet slurry into a precision test instrument capable of delivering reliable scientific data.
Summary Table:
| Feature | Impact on In2O3/C Electrode Preparation | Benefit for Electrochemical Evaluation |
|---|---|---|
| Solvent Removal | Eliminates trapped liquids from porous structures | Prevents parasitic side reactions with electrolyte |
| Adhesion Support | Binders shrink and harden uniformly in vacuum | Prevents material delamination during battery cycling |
| Moisture Control | Lowers boiling points for deep-layer dehydration | Increases Coulombic efficiency and data reliability |
| Temperature Balance | Allows drying without thermal degradation of binders | Maintains material morphology and integrity |
Elevate Your Battery Research with KINTEK
Precision in electrode preparation starts with the right thermal environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Muffle, and Tube furnace systems specifically designed to meet the rigorous demands of battery material research.
Whether you are refining In2O3/C composites or developing next-generation energy storage, our customizable high-temperature solutions ensure the chemical purity and mechanical stability your lab requires. Contact KINTEK today to optimize your drying protocol!
Visual Guide
References
- Wenhe Xie, Xiaolei Sun. Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries. DOI: 10.3390/inorganics12120336
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How do continuous furnaces enhance production efficiency? Boost Throughput & Cut Costs
- How does vacuum brazing contribute to environmental and safety advantages? Discover Cleaner, Safer Metal Joining
- What is the purpose of using a vacuum drying oven for carbon microspheres? Optimize Your Material Activation
- How does vacuum annealing and tempering improve material properties? Enhance Strength, Purity, and Durability
- What role does a high-temperature vacuum furnace play in the synthesis of LaTiOC/NdTiOC? Master Heteroanionic Materials
- How does vacuum carburizing ensure stable carburizing quality? Achieve Precise, Repeatable Heat Treatment
- What are the different types of crucible furnaces based on how molten metal is removed? A Guide to Lift-Out, Bale-Out, and Tilting Designs
- How does the vacuum environment contribute to medical device manufacturing? Ensure Purity and Precision for Patient Safety



















