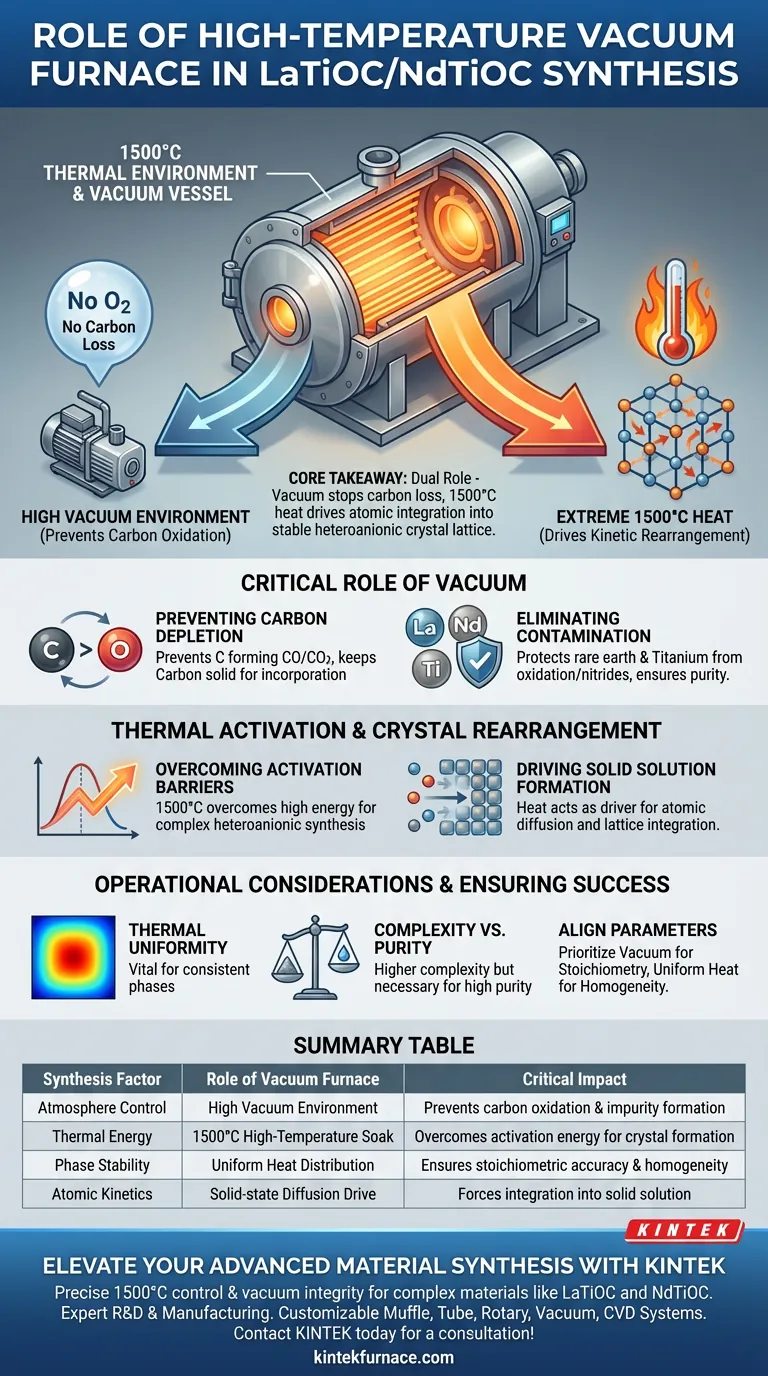
A high-temperature vacuum furnace serves as the essential reaction vessel, providing the extreme 1500°C thermal environment and controlled atmosphere required to synthesize Lanthanum or Neodymium-based Titanium Oxycarbide (LaTiOC/NdTiOC). This equipment performs two simultaneous functions: it supplies the kinetic energy to force ionic rearrangement and maintains a vacuum to prevent the constituent carbon from oxidizing and escaping the material.
Core Takeaway: The furnace plays a dual role: its vacuum prevents carbon loss through oxidation, while the 1500°C heat drives the kinetic rearrangement necessary to integrate titanium, oxygen, carbon, and rare earth ions into a single, stable heteroanionic crystal lattice.

The Critical Role of the Vacuum Environment
Preventing Carbon Depletion
The synthesis of oxycarbides requires maintaining a precise stoichiometric ratio between oxygen and carbon within the material.
At high temperatures, carbon is highly reactive with atmospheric oxygen, readily forming CO or CO2 gas.
The vacuum atmosphere removes ambient oxygen, ensuring the carbon remains in the solid phase to be incorporated into the crystal structure rather than burning off.
Eliminating Contamination
Beyond carbon preservation, the vacuum environment protects the rare earth elements (Lanthanum and Neodymium) and Titanium.
These metals are susceptible to oxidation or nitride formation if exposed to air at high temperatures.
A high vacuum ensures that the only elements reacting are the intended precursors, securing the chemical purity of the final product.
Thermal Activation and Crystal Rearrangement
Overcoming Activation Energy Barriers
Creating a heteroanionic material—where two different anions (oxygen and carbon) share the same lattice—requires significant energy.
The furnace provides an intense heat of 1500°C to overcome the high activation energy barriers associated with this complex synthesis.
Without this extreme temperature, the precursors would remain inert or form incomplete intermediate phases.
Driving Solid Solution Formation
Heat acts as the driver for atomic diffusion.
At 1500°C, the ions gain the kinetic energy necessary to migrate and rearrange themselves within the solid state.
This thermal drive forces the titanium, rare earth ions, oxygen, and carbon to integrate into a uniform solid solution, establishing the specific crystal lattice of LaTiOC or NdTiOC.
Operational Considerations and Trade-offs
The Necessity of Thermal Uniformity
While reaching 1500°C is the primary requirement, the uniformity of that heat is equally vital.
Inconsistent heating zones can lead to a mixture of phases, where some portions of the sample are fully reacted while others are not.
High-quality furnaces mitigate this by ensuring the temperature profile is consistent across the entire sample zone.
Complexity vs. Purity
Using a high-temperature vacuum furnace introduces higher operational complexity and cost compared to standard atmospheric furnaces.
However, this is a necessary trade-off.
Attempting to synthesize these specific oxycarbides in an inert gas flow (like Argon) without high vacuum capabilities may still pose a risk of trace oxidation or insufficient purity for high-performance applications.
Ensuring Synthesis Success
To achieve high-quality LaTiOC or NdTiOC, align your furnace parameters with your specific goals:
- If your primary focus is stoichiometric accuracy: Prioritize a high-vacuum environment to strictly prevent carbon loss, as even minor oxidation will shift the anion ratio.
- If your primary focus is phase homogeneity: Ensure the furnace can maintain a stable, uniform 1500°C soak for the duration required to complete the solid-state diffusion.
By strictly controlling both the vacuum atmosphere and the thermal kinetic energy, you transform raw precursors into a precise, structurally sound heteroanionic material.
Summary Table:
| Synthesis Factor | Role of Vacuum Furnace | Critical Impact |
|---|---|---|
| Atmosphere Control | High Vacuum Environment | Prevents carbon oxidation and impurity formation (nitrides) |
| Thermal Energy | 1500°C High-Temperature Soak | Overcomes activation energy for heteroanionic crystal formation |
| Phase Stability | Uniform Heat Distribution | Ensures stoichiometric accuracy and crystal lattice homogeneity |
| Atomic Kinetics | Solid-state Diffusion Drive | Forces integration of rare earth ions, Ti, O, and C into solid solution |
Elevate Your Advanced Material Synthesis with KINTEK
Precise control over 1500°C thermal profiles and vacuum integrity is non-negotiable for synthesizing complex heteroanionic materials like LaTiOC and NdTiOC. KINTEK provides industry-leading high-temperature vacuum systems designed to prevent carbon depletion and ensure phase purity.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique research or production requirements. Partner with us to achieve the stoichiometric accuracy and phase homogeneity your high-performance applications demand.
Ready to optimize your synthesis process? Contact KINTEK today for a consultation!
Visual Guide
References
- Yathavan Subramanian, Abul Kalam Azad. Heteroanionic synthesis of lanthanum/neodymium-based titanium oxycarbide: a novel approach with multiple objectives for clean energy and pollutant-free environment. DOI: 10.1093/ce/zkae081
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is a high-temperature vacuum furnace with argon protection required for sintering NiTi/HA? Ensure Phase Purity
- How does a retort furnace compare to a vacuum furnace? Choose the Right Heat Treatment for Your Materials
- How does the ultra-low oxygen environment of vacuum sintering affect titanium composites? Unlock Advanced Phase Control
- Why is a high-temperature vacuum annealing furnace essential for graphene aerogels? Unlock Ultimate Conductivity
- Why is a high-precision vacuum oven necessary for RGO/PI composite films? Ensure Defect-Free Graded Heat Treatment
- What is a continuous vacuum furnace and how does it differ from traditional batch furnaces? Boost Your High-Volume Production Efficiency
- What is the primary function of a vacuum graphite furnace? Achieve Extreme-Temperature Material Purity
- What materials can be brazed in a furnace? Unlock Versatile Joining for Metals and Ceramics



















