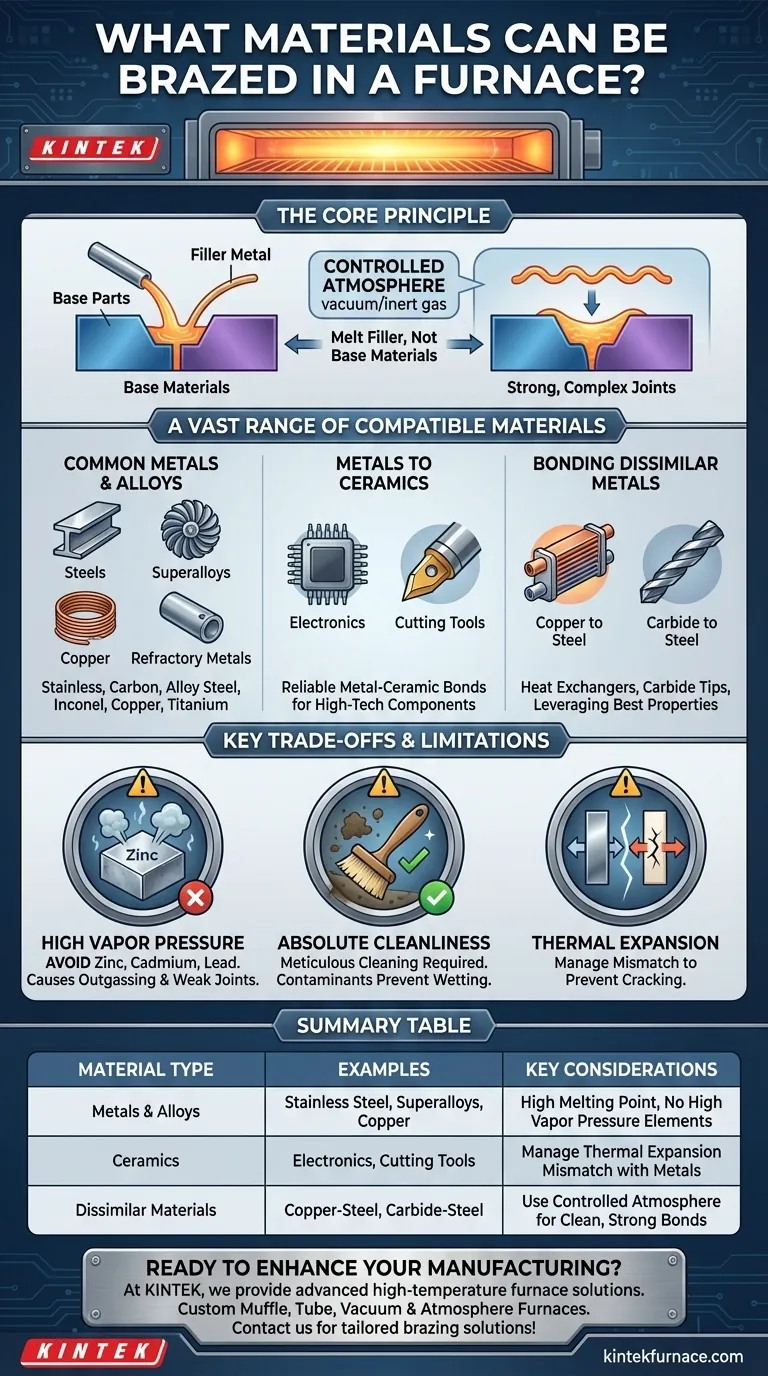
In furnace brazing, a vast range of materials can be successfully joined. The process is not limited to similar metals; it excels at bonding dissimilar metals and even metals to ceramics. Common materials include various steels, superalloys, copper, and titanium, with the primary limitation being the avoidance of base materials or fillers containing elements with high vapor pressure, such as zinc or cadmium.
Furnace brazing's versatility comes from its core principle: using a controlled atmosphere to melt a filler metal that bonds base materials without melting them. This allows for the creation of strong, complex joints between a wide array of metals, alloys, and ceramics, making it one of the most adaptable joining technologies available.

The Principles of Material Compatibility
Furnace brazing is a highly precise process. Understanding how it interacts with different materials is key to leveraging its full potential. The compatibility is determined by the interplay between the base materials, the filler metal, and the furnace atmosphere.
The Role of Base Materials
The fundamental rule is that the melting point of the base materials must be significantly higher than the melting point of the filler metal. The process heats the entire assembly, and the base parts must remain solid and stable as the filler becomes liquid.
This is why materials like stainless steels, nickel-based superalloys, copper alloys, and even ceramics are excellent candidates. Their high melting temperatures allow for a wide selection of filler metals.
The Critical Function of the Filler Metal
The filler metal is the agent that creates the bond. It is chosen based on its own melting point, its compatibility with the base materials, and the desired properties of the final joint (e.g., strength, corrosion resistance).
When heated past its melting point, the filler metal is drawn into the tight-fitting gap between the base materials through capillary action. Upon cooling, it forms a strong, permanent metallurgical bond.
The Power of a Controlled Atmosphere
Furnace brazing is almost always performed in a controlled atmosphere, such as a vacuum or an inert gas environment. This prevents the oxidation of the base and filler materials at elevated temperatures.
This control is what enables the joining of reactive metals like titanium or the creation of exceptionally clean joints required for medical implants and aerospace components.
A Guide to Compatible Materials
The process is renowned for its ability to join materials that are difficult or impossible to weld. This flexibility opens up design possibilities across numerous industries.
Common Metals and Alloys
A wide spectrum of metals can be brazed. This includes:
- Steels: Stainless steel, carbon steel, and alloy steels.
- Superalloys: Nickel-based (e.g., Inconel) and cobalt-based alloys used in aerospace and turbines.
- Copper and Copper Alloys: Valued for their thermal and electrical conductivity.
- Refractory Metals: Such as titanium, which requires a vacuum environment.
Joining Metals to Ceramics
Furnace brazing is one of the few reliable methods for creating a strong bond between a metal and a ceramic. This is critical for manufacturing components like electronic packages or cutting tools where the properties of both materials are required.
Bonding Dissimilar Metals
The process excels at joining different types of metals, such as copper to steel in heat exchangers or carbide tips to steel bodies in cutting tools. This allows engineers to design components that leverage the best properties of multiple materials in a single assembly.
Understanding the Trade-offs and Limitations
While incredibly versatile, furnace brazing has specific material constraints that are critical to understand for successful application. Ignoring these can lead to failed joints and contaminated equipment.
The High Vapor Pressure Constraint
The most significant limitation is that materials containing elements with high vapor pressure must be avoided. In the vacuum of a brazing furnace, elements like zinc, cadmium, lead, and magnesium will "boil off" or outgas from the parent material.
This outgassing can contaminate the furnace, interfere with the brazing process, and create porous, weak joints. This is why common alloys like brass (containing zinc) are generally unsuitable for vacuum furnace brazing.
The Requirement for Absolute Cleanliness
All components must be meticulously cleaned before being placed in the furnace. Any oils, grease, oxides, or other contaminants on the surface will prevent the filler metal from wetting the material and flowing properly, resulting in a failed bond.
Mismatch in Thermal Expansion
When joining dissimilar materials, particularly metals to ceramics, their different rates of thermal expansion and contraction must be managed. A significant mismatch can induce stress in the joint as it cools, potentially leading to cracks or failure. This often requires careful joint design and selection of an appropriate ductile filler metal.
Choosing the Right Materials for Your Application
Your final material selection will depend entirely on the performance requirements of the finished part.
- If your primary focus is high-temperature performance (e.g., aerospace): Join stainless steels or superalloys using nickel or gold-based filler metals for superior strength and corrosion resistance.
- If your primary focus is joining dissimilar materials (e.g., electronics): Use furnace brazing for robust metal-to-ceramic bonds, but carefully manage thermal expansion differences.
- If your primary focus is complex assemblies (e.g., heat exchangers): Leverage the ability to join multiple joints simultaneously on base materials like steel, copper, and aluminum.
- If your primary focus is tool and die manufacturing: Braze carbide sections to steel bodies to create tools with exceptional hardness and wear resistance.
By understanding these material principles, you can confidently apply furnace brazing to solve a wide range of complex manufacturing challenges.
Summary Table:
| Material Type | Examples | Key Considerations |
|---|---|---|
| Metals & Alloys | Stainless steel, superalloys (e.g., Inconel), copper, titanium | High melting point, avoid high vapor pressure elements (e.g., zinc, cadmium) |
| Ceramics | Various ceramics for electronics, cutting tools | Manage thermal expansion mismatch with metals |
| Dissimilar Materials | Copper to steel, carbide to steel | Use controlled atmosphere for clean, strong bonds |
Ready to enhance your manufacturing with precise furnace brazing solutions? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we meet your unique experimental and production needs, whether you're in aerospace, electronics, or tool manufacturing. Contact us today to discuss how our tailored brazing furnaces can deliver superior performance and reliability for your projects!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does a mixed gas flow control system maintain stability during high-temperature nitriding? Precision Gas Ratios
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- What is inert gas technology used for in high-temperature atmosphere vacuum furnaces? Protect Materials and Speed Up Cooling
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process



















