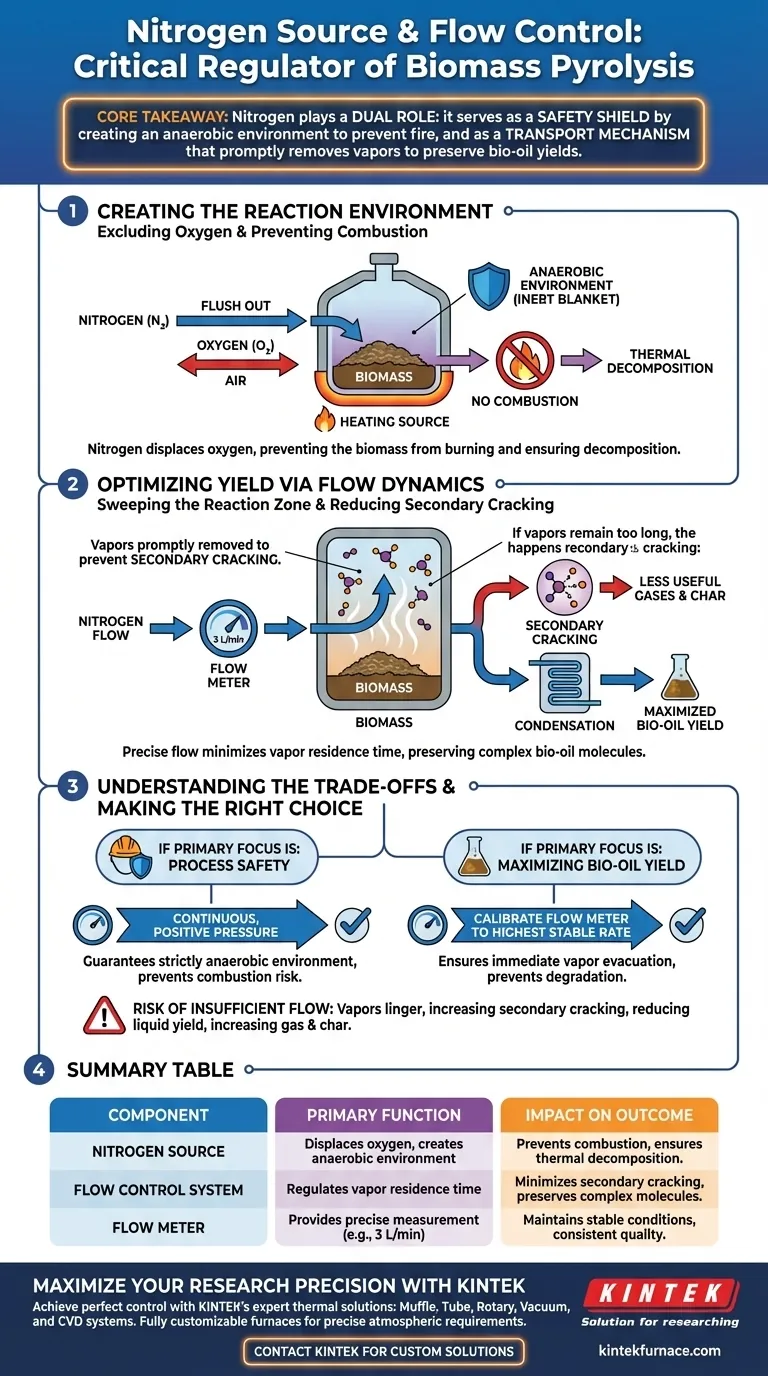
The nitrogen source and flow control system function as the critical environmental regulator for the biomass pyrolysis process. Nitrogen acts as an inert carrier gas that displaces oxygen to prevent combustion, while the flow control system regulates the speed at which volatile vapors are swept out of the reactor. Together, they ensure the biomass undergoes thermal decomposition rather than burning, directly protecting the chemical integrity of the desired bio-oil.
Core Takeaway: Nitrogen plays a dual role: it serves as a safety shield by creating an anaerobic environment to prevent fire, and as a transport mechanism that promptly removes vapors to preserve bio-oil yields.

Creating the Reaction Environment
Excluding Oxygen
Pyrolysis is defined as thermal decomposition in the absence of oxygen.
Nitrogen acts as an inert "blanket," effectively flushing air out of the system. This creates the strictly oxygen-limited or anaerobic environment required for the reaction to proceed correctly.
Preventing Combustion
Biomass is heated to very high temperatures during pyrolysis.
If oxygen were present, the biomass would simply ignite and burn (combustion) rather than decomposing into useful fuels. The nitrogen source prevents this by removing the oxidizer necessary for fire.
Optimizing Yield via Flow Dynamics
Sweeping the Reaction Zone
As the biomass heats up, it releases volatile gases.
The nitrogen flow physically carries these gases away from the hot reaction zone. This transport mechanism is managed by a flow meter to ensure a consistent volume, such as 3 liters per minute.
Reducing Secondary Cracking
Timing is critical in pyrolysis.
If hot vapors remain in the reactor too long, they undergo secondary cracking reactions. This breaks down the valuable, complex molecules needed for bio-oil into smaller, less useful gas molecules.
Maximizing Bio-Oil Production
The flow control system minimizes the "residence time" of the vapors.
By promptly removing these gases before they can degrade, the system preserves the chemical structure of the volatiles. This directly leads to an increased yield of liquid bio-oil upon condensation.
Understanding the Trade-offs
The Risk of Insufficient Flow
If the nitrogen flow rate is too low, vapors linger in the high-temperature zone.
This increases the likelihood of secondary cracking, which reduces liquid yield and increases the production of non-condensable gases and char.
The Precision of Control
Flow is not a "set it and forget it" variable; it requires precise measurement via a flow meter.
The rate must be high enough to clear vapors instantly, but controlled enough to maintain stable reactor conditions. An arbitrary flow rate can disrupt the thermal balance or fail to evacuate vapors efficiently.
Making the Right Choice for Your Goal
To optimize your pyrolysis setup, you must view the nitrogen system as a variable that directly dictates product quality.
- If your primary focus is Process Safety: Ensure the nitrogen source provides a continuous, positive pressure to guarantee a strictly anaerobic environment, preventing any risk of combustion.
- If your primary focus is Maximizing Bio-Oil Yield: Calibrate your flow meter to the highest rate that allows for stable heating, ensuring volatile gases are evacuated immediately to prevent degradation.
The precise management of nitrogen flow is the difference between generating high-grade fuel and producing low-value gas.
Summary Table:
| Component | Primary Function | Impact on Pyrolysis Outcome |
|---|---|---|
| Nitrogen Source | Displaces oxygen to create an anaerobic environment | Prevents combustion; ensures thermal decomposition instead of burning. |
| Flow Control System | Regulates the residence time of volatile vapors | Minimizes secondary cracking; preserves complex molecules for bio-oil. |
| Flow Meter | Provides precise measurement of gas volume (e.g., 3 L/min) | Maintains stable reactor conditions and consistent product quality. |
Maximize Your Research Precision with KINTEK
Achieve perfect control over your pyrolysis environment with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to handle precise atmospheric requirements.
Whether you need to optimize bio-oil yields or ensure a strictly anaerobic reaction, our laboratory high-temperature furnaces are fully customizable to meet your unique research needs.
Ready to elevate your lab's efficiency? Contact KINTEK today to discuss your custom furnace solution!
Visual Guide
References
- Haniif Prasetiawan, R Fitrah. The Effect of Raw Material Composition and Pyrolysis Temperature on The Characteristics of Bio-Oil from the Pyrolysis of Sawdust and Sugar Cane Bagasse Mixture. DOI: 10.1051/e3sconf/202564803007
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Spark Plasma Sintering SPS Furnace
People Also Ask
- How do surface states affect lithium carbonate crystal morphology? Controlling Nucleation for Superior Particle Shape
- What role does the vitreous carbon foam framework play in PTTM? Unlock Biomimetic Dental Implant Precision
- What are the advantages of using a vacuum drying oven for ZIF67/MXene? Protect Your Composite Integrity
- What is the role of a TG-FTIR-MS coupled system in 5AT and NaIO4 analysis? Master Thermal Decomposition Insights
- What are the advantages of using a multimode microwave applicator for alloy cladding? Rapid, Volumetric Internal Heat
- What is the purpose of using a spiral grain selector? Achieving Single-Crystal Precision in Metal Castings
- Why Use the Modified Two-Temperature Synthesis for ZnGeP2? Ensure Safety and Material Quality
- How do laboratory high-temperature resistance furnaces simulate industrial production processes for 01YUT steel?



















