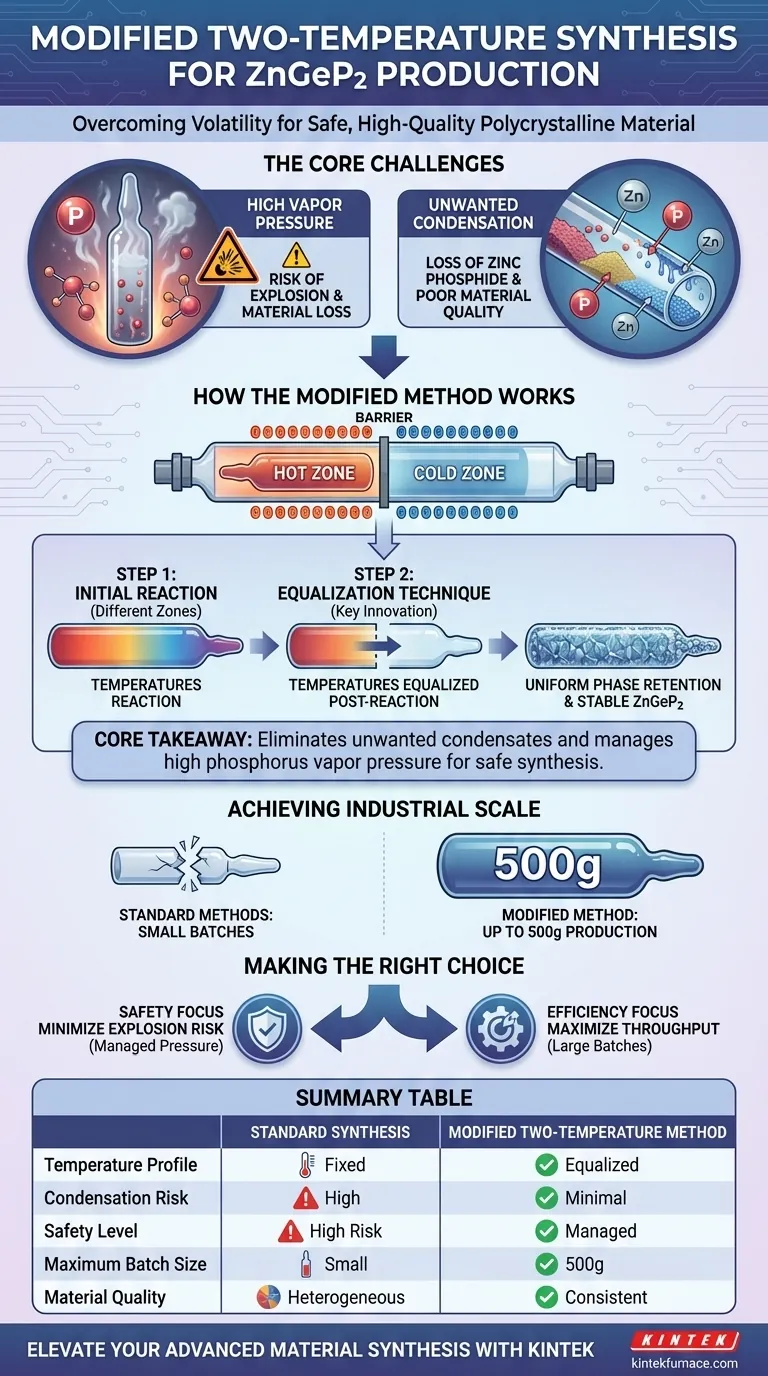
The modified two-temperature synthesis method is primarily utilized to manage the volatility of reactive components and ensure the safe production of high-quality material. It is specifically designed to prevent the condensation of binary compounds like zinc phosphide and to mitigate the dangers associated with high internal pressure during the synthesis of Zinc Germanium Phosphide (ZnGeP2).
Core Takeaway By equalizing temperatures between hot and cold zones post-reaction, this method eliminates the formation of unwanted condensates and manages high phosphorus vapor pressure. This innovation enables the safe synthesis of batches up to 500g, significantly improving production efficiency compared to traditional techniques.

The Core Challenges of ZnGeP2 Production
Managing High Vapor Pressure
The synthesis of Zinc Germanium Phosphide involves volatile elements, particularly Phosphorus.
At the high temperatures required for reaction, Phosphorus generates significant vapor pressure.
Without a specialized method to manage this pressure, there is a high risk of ampoule explosions, posing a major safety hazard and loss of material.
Preventing Unwanted Condensation
A critical issue in standard synthesis attempts is the behavior of intermediate compounds.
Volatile binary phosphides, specifically zinc phosphide, have a tendency to condense out of the reaction mixture.
If these components condense separately, they do not participate correctly in forming the final ternary compound, resulting in poor material quality.
How the Modified Method Works
The Equalization Technique
The defining feature of this "modified" approach is the precise control of thermal profiles.
After the initial reaction, the method involves equalizing the temperatures of both the cold and hot zones of the furnace.
This thermal balance ensures that volatile components remain in the correct phase to react, rather than condensing prematurely in cooler regions.
Achieving Industrial Scale
Standard synthesis methods are often restricted to small batches due to the volatility and pressure risks mentioned above.
The modified two-temperature method allows for the preparation of significantly larger volumes, up to 500g in a single process.
This capability transforms the process from a laboratory curiosity into an efficient production method.
Critical Risks and Considerations
The Consequence of Thermal Imbalance
It is vital to understand that the success of this method hinges on the temperature equalization step.
If the temperature difference between zones is maintained rather than equalized, zinc phosphide will likely condense.
This leads to a heterogeneous mixture rather than the desired polycrystalline ZnGeP2.
Safety Margins
While this method reduces risk, the handling of high-pressure phosphorus vapor always requires caution.
The method mitigates explosion risks, but the integrity of the ampoule and precise temperature control remain the primary safeguards against catastrophic failure.
Making the Right Choice for Your Goal
This method is the definitive choice when scaling up production while maintaining stoichiometry.
- If your primary focus is Safety: This method is essential to minimize the risk of ampoule explosions caused by unmanaged phosphorus vapor pressure.
- If your primary focus is Efficiency: Adopt this technique to maximize throughput, allowing for single-batch production of up to 500g of material.
Ultimately, this method provides the necessary thermal control to convert high-risk volatile elements into stable, high-quality polycrystalline material.
Summary Table:
| Feature | Standard Synthesis | Modified Two-Temperature Method |
|---|---|---|
| Temperature Profile | Fixed Hot/Cold Zones | Equalized Hot/Cold Zones Post-Reaction |
| Condensation Risk | High (Zinc Phosphide loss) | Minimal (Uniform phase retention) |
| Safety Level | High Risk of Ampoule Explosion | Managed Phosphorus Vapor Pressure |
| Maximum Batch Size | Small/Limited | Up to 500g |
| Material Quality | Often Heterogeneous | Consistent Polycrystalline ZnGeP2 |
Elevate Your Advanced Material Synthesis with KINTEK
Precise thermal management is the difference between a successful batch and a catastrophic failure. Backed by expert R&D and world-class manufacturing, KINTEK offers specialized Muffle, Tube, and Vacuum systems designed to handle the rigorous demands of volatile compound synthesis.
Whether you are scaling Zinc Germanium Phosphide (ZnGeP2) production or developing custom semiconductor materials, our high-temperature furnaces are fully customizable to meet your unique safety and stoichiometric requirements.
Ready to optimize your lab's throughput and safety? Contact our engineering experts today to find the perfect thermal solution for your research and production needs.
Visual Guide
References
- Alexey Lysenko, Alexey Olshukov. Band-like Inhomogeneity in Bulk ZnGeP2 Crystals, and Composition and Influence on Optical Properties. DOI: 10.3390/cryst15040382
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- How do high-precision industrial furnaces contribute to thermal stability research in cement composites?
- Why is high-precision temperature control at 800 °C critical for BCMoMn catalyst heterostructures?
- What is the significance of high vacuum base pressure in MoS2 sputtering? Ensuring Film Purity and Stoichiometry
- What roles does a laboratory oven play in biochar production? Enhance Efficiency and Accuracy in Thermal Processing
- What role do high-temperature industrial furnaces play in the pretreatment of spodumene for lithium extraction?
- What is the purpose of using a vacuum dryer for PU and AlN composite sheets? Enhance Thermal & Structural Integrity
- How does a precision carbon dioxide gas flow control system influence the precipitation of high-purity lithium carbonate?



















