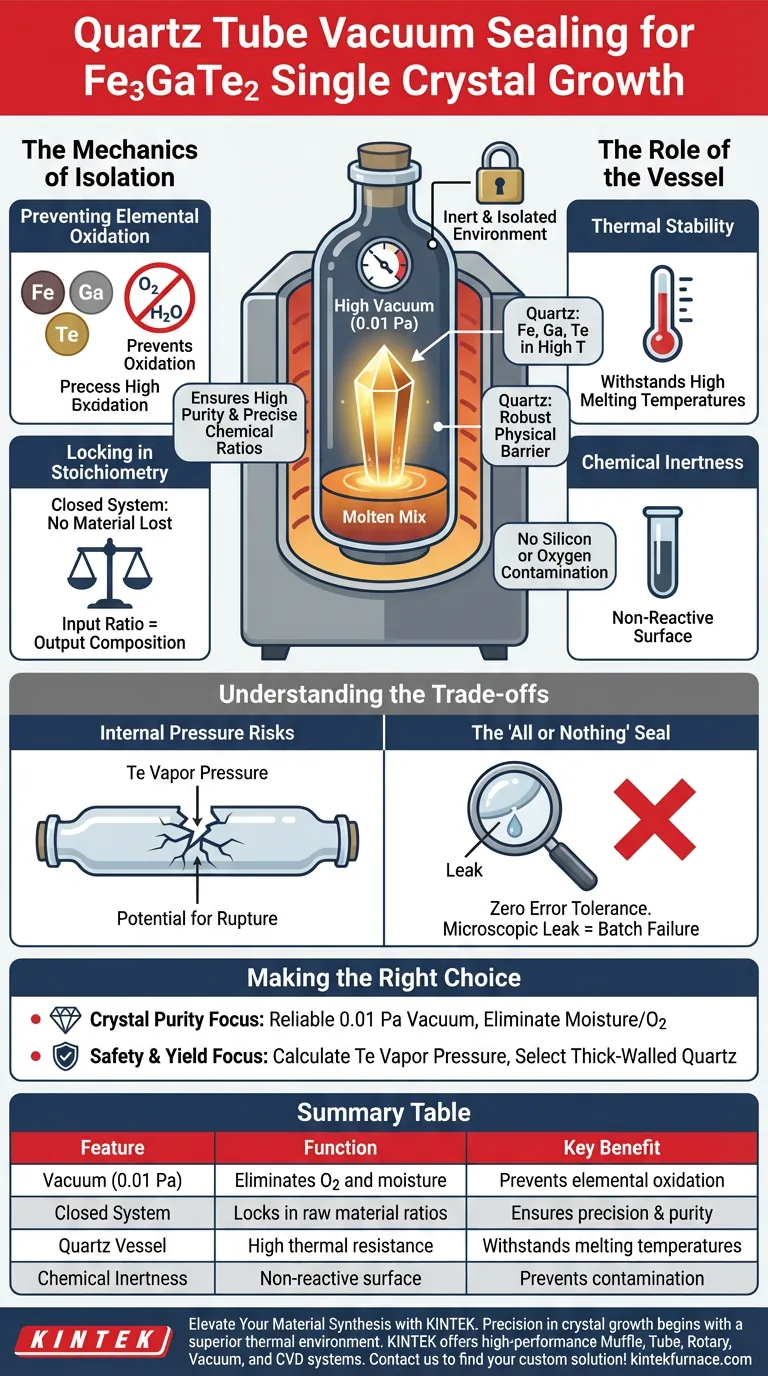
The primary function of quartz tube vacuum sealing is to create an inert, isolated environment essential for chemical stability. Specifically for growing $Fe_3GaTe_2$ single crystals via the self-flux method, sealing the raw materials at a high vacuum (0.01 Pa) prevents the oxidation of iron, gallium, and tellurium during the high-temperature melting process, ensuring the final crystal maintains high purity and precise chemical ratios.
By eliminating oxygen and moisture, the vacuum-sealed quartz tube acts as a strict control vessel that forces the elements to react with each other rather than the atmosphere. This isolation is the deciding factor in achieving the correct stoichiometric balance required for high-quality single crystal formation.
The Mechanics of Isolation
Preventing Elemental Oxidation
At the high temperatures required for crystal growth, raw materials such as Iron (Fe), Gallium (Ga), and Tellurium (Te) become highly reactive.
Exposed to even trace amounts of air, these elements will rapidly form oxides.
The quartz tube, evacuated to 0.01 Pa, removes these atmospheric contaminants, ensuring the raw materials remain in their metallic elemental forms.
Locking in Stoichiometry
The quality of a single crystal depends on maintaining an exact ratio of atoms (stoichiometry).
If a portion of the Iron or Gallium is lost to oxidation, the ratio shifts, potentially leading to impurities or structural defects in the crystal lattice.
Vacuum sealing creates a closed system where no material enters or leaves, guaranteeing that the input ratio matches the output composition.
The Role of the Vessel
Thermal Stability
The growth process requires bringing the mixture to a molten state.
Quartz is utilized because it offers exceptional thermal resistance, maintaining its structural integrity at the processing temperatures required to melt the flux and solute.
It serves as a robust physical barrier that withstands the thermal stress of the furnace without degrading.
Chemical Inertness
Beyond temperature resistance, the reaction vessel must not contaminate the melt.
Quartz is chemically inert regarding the specific reactants ($Fe$, $Ga$, $Te$) used in this process.
This ensures that the tube acts solely as a container, preventing silicon or oxygen from the tube wall from leaching into the developing crystal.
Understanding the Trade-offs
Internal Pressure Risks
While the vacuum seal protects against external air, it creates a closed pressure environment internally.
Volatile elements like Tellurium can generate significant vapor pressure when heated.
If the quartz tube is flawed or the walls are too thin, this internal pressure can cause the vessel to rupture during synthesis.
The "All or Nothing" Seal
The success of this method relies entirely on the perfection of the vacuum seal.
Unlike open systems where minor fluctuations might be tolerated, a vacuum-sealed tube allows for zero error.
A microscopic leak or an imperfect seal at 0.01 Pa renders the entire process void, as atmospheric contamination will immediately compromise the purity of the batch.
Making the Right Choice for Your Goal
To ensure the successful growth of $Fe_3GaTe_2$, you must prioritize the integrity of the sealing process.
- If your primary focus is crystal purity: Ensure your vacuum system reliably achieves 0.01 Pa or lower to fully eliminate moisture and oxygen pockets before sealing.
- If your primary focus is safety and yield: Calculate the expected vapor pressure of Tellurium at your maximum temperature and select quartz tubing with sufficient wall thickness to withstand the stress.
The difference between a high-grade single crystal and a contaminated sample effectively comes down to the quality of your vacuum environment.
Summary Table:
| Feature | Function in Fe3GaTe2 Growth | Key Benefit |
|---|---|---|
| Vacuum (0.01 Pa) | Eliminates O2 and moisture | Prevents elemental oxidation of Fe, Ga, and Te |
| Closed System | Locks in raw material ratios | Ensures precise stoichiometry and crystal purity |
| Quartz Vessel | High thermal resistance | Withstands melting temperatures without degradation |
| Chemical Inertness | Non-reactive surface | Prevents vessel-to-sample contamination |
Elevate Your Material Synthesis with KINTEK
Precision in crystal growth begins with a superior thermal environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for advanced laboratory needs. Whether you are growing $Fe_3GaTe_2$ single crystals or developing new alloys, our customizable high-temp furnaces provide the vacuum integrity and thermal stability essential for your success.
Ready to optimize your lab's performance? Contact us today to find your custom solution!
Visual Guide
References
- Ki‐Hoon Son, Hyejin Ryu. Persistent ferromagnetic ground state in pristine and Ni-doped Fe3GaTe2 flakes. DOI: 10.1186/s40580-024-00458-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
People Also Ask
- How does a Tube CVD furnace facilitate the in-situ synthesis of CNT/Cu composite powders? Achieve Superior Material Bonds
- How are horizontal furnaces utilized in the automotive sector? Boost Component Durability and Efficiency
- Why is it necessary to use a tube furnace for air oxidation of the 3D copper framework? Master Lithiophilic Interfaces
- What are the main components of a tube furnace? Essential Parts for Precise High-Temperature Processing
- What is the function of a rotameter in a tube furnace? Master Gas Flow Precision for Reliable Thermal Analysis
- What are the benefits of quartz tube furnaces? Achieve Purity and Visibility in High-Temp Processes
- How does an alumina-lined vertical tube furnace provide a stable environment for corrosion experiments? Get Expert Data
- Why is annealing in a tube furnace essential for rGO-NiO-ZnO-400? Optimize Your Catalyst Synthesis



















