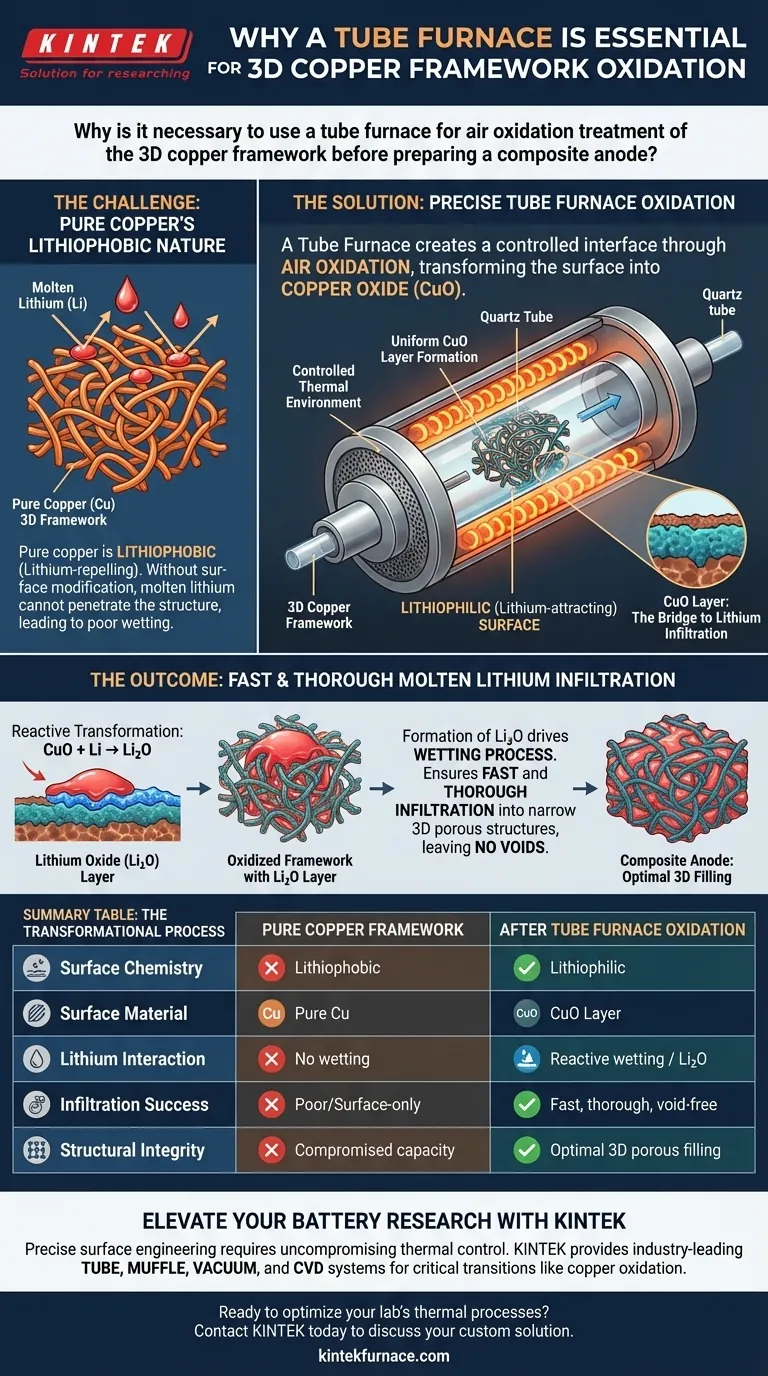
The primary purpose of using a tube furnace is to create a precise, controlled interface. Specifically, it allows for the air oxidation of the 3D copper framework to form a uniform layer of copper oxide (CuO). This oxidation step is the critical enabler that allows molten lithium to bond with and penetrate the copper structure during subsequent processing.
The central challenge in this process is that pure copper naturally repels molten lithium. The tube furnace treatment solves this by chemically altering the surface from lithiophobic (lithium-repelling) to lithiophilic (lithium-attracting), ensuring the anode structure can actually be filled.

Overcoming the Surface Chemistry Barrier
The Inherent Problem with Pure Copper
On its own, a pure copper framework presents a significant manufacturing hurdle. It is inherently lithiophobic, meaning it resists wetting by molten lithium. Without modification, molten lithium would simply sit on the surface rather than penetrating the structure.
The Role of Copper Oxide (CuO)
The tube furnace provides the thermal environment necessary to oxidize the copper surface in air. This transforms the outer layer of the copper strands into copper oxide (CuO). Unlike pure copper, this oxide layer has chemical properties that are favorable for interaction with lithium.
Creating a Lithiophilic Interface
The presence of CuO is not the final goal, but the bridge to it. This layer is described as lithiophilic, creating the necessary surface tension conditions to invite contact with lithium.
Facilitating Molten Lithium Infiltration
The Reactive Transformation to Li2O
When the oxidized framework contacts molten lithium, a chemical reaction occurs. The copper oxide layer reacts with the lithium to form a lithium oxide (Li2O) layer. This newly formed Li2O layer is the active agent that drives the wetting process.
Ensuring Fast and Thorough Filling
The formation of Li2O dramatically changes the fluid dynamics of the system. It enables fast and thorough infiltration of the molten lithium. This is particularly vital for navigating the "narrow 3D porous structure" of the framework, ensuring no voids are left behind.
Understanding the Necessity of Control
Precision in Layer Formation
You might ask why a tube furnace is used specifically, rather than a simple open flame or oven. The key word in the engineering requirement is a "controlled layer."
Avoiding Inconsistent Oxidation
A tube furnace provides a stable thermal profile. If the oxidation is uneven or uncontrolled, the conversion to CuO will be inconsistent. This would lead to patchy infiltration, leaving parts of the 3D framework unfilled and compromising the anode's final capacity.
Making the Right Choice for Your Goal
To maximize the effectiveness of your composite anode preparation, consider these factors regarding the oxidation step:
- If your primary focus is wetting speed: Ensure the oxidation layer is sufficient to generate a continuous Li2O interface, as this reaction drives the capillary action needed for rapid filling.
- If your primary focus is structural density: Prioritize the uniformity of the oxidation in the tube furnace to ensure molten lithium reaches the deepest, narrowest pores of the 3D framework.
The tube furnace is not just a heating step; it is a surface engineering tool that converts a hostile substrate into a receptive host for lithium.
Summary Table:
| Process Feature | Pure Copper Framework | After Tube Furnace Oxidation |
|---|---|---|
| Surface Chemistry | Lithiophobic (Lithium-repelling) | Lithiophilic (Lithium-attracting) |
| Surface Material | Pure Cu | Copper Oxide (CuO) Layer |
| Lithium Interaction | No wetting/High surface tension | Reactive wetting (Forms Li2O) |
| Infiltration Success | Poor/Surface-only | Fast, thorough, and void-free |
| Structural Integrity | Compromised capacity | Optimal 3D porous filling |
Elevate Your Battery Research with KINTEK
Precise surface engineering requires uncompromising thermal control. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed to facilitate critical transitions like copper oxidation with absolute uniformity.
Backed by expert R&D and manufacturing, our high-temp furnaces are fully customizable to meet the unique demands of your composite anode development and material science breakthroughs.
Ready to optimize your lab's thermal processes? Contact KINTEK today to discuss your custom solution.
Visual Guide
References
- Inyeong Yang, Sanha Kim. Ultrathin 3D Cu/Li Composite with Enhanced Li Utilization for High Energy Density Li‐Metal Battery Anodes. DOI: 10.1002/smll.202501629
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is a rotary tube furnace? Achieve Superior Uniformity for Powders & Granules
- What are the common applications of alumina tube furnaces? Unlock Precision in Materials Processing
- What heating temperatures can tube furnaces achieve? Unlock Precision Up to 1800°C for Your Lab
- What is the function of a tube furnace in catalyst annealing? Unlock L10 Ordered Structures for Peak Performance
- Why is a high-temperature tube furnace required for the post-treatment of composite anode materials in argon?
- What is the role of a laboratory tube furnace in the heat treatment of Zr-2.5%Nb pressure tube samples? (550°C-800°C)
- Why is a tube furnace required for the calcination of TiO2 in an H2/Ar mixed atmosphere? Engineering TiO2-X Defects
- What are the advantages of a one-zone tube furnace for MoS2 synthesis? Ensure Uniformity and Repeatability



















