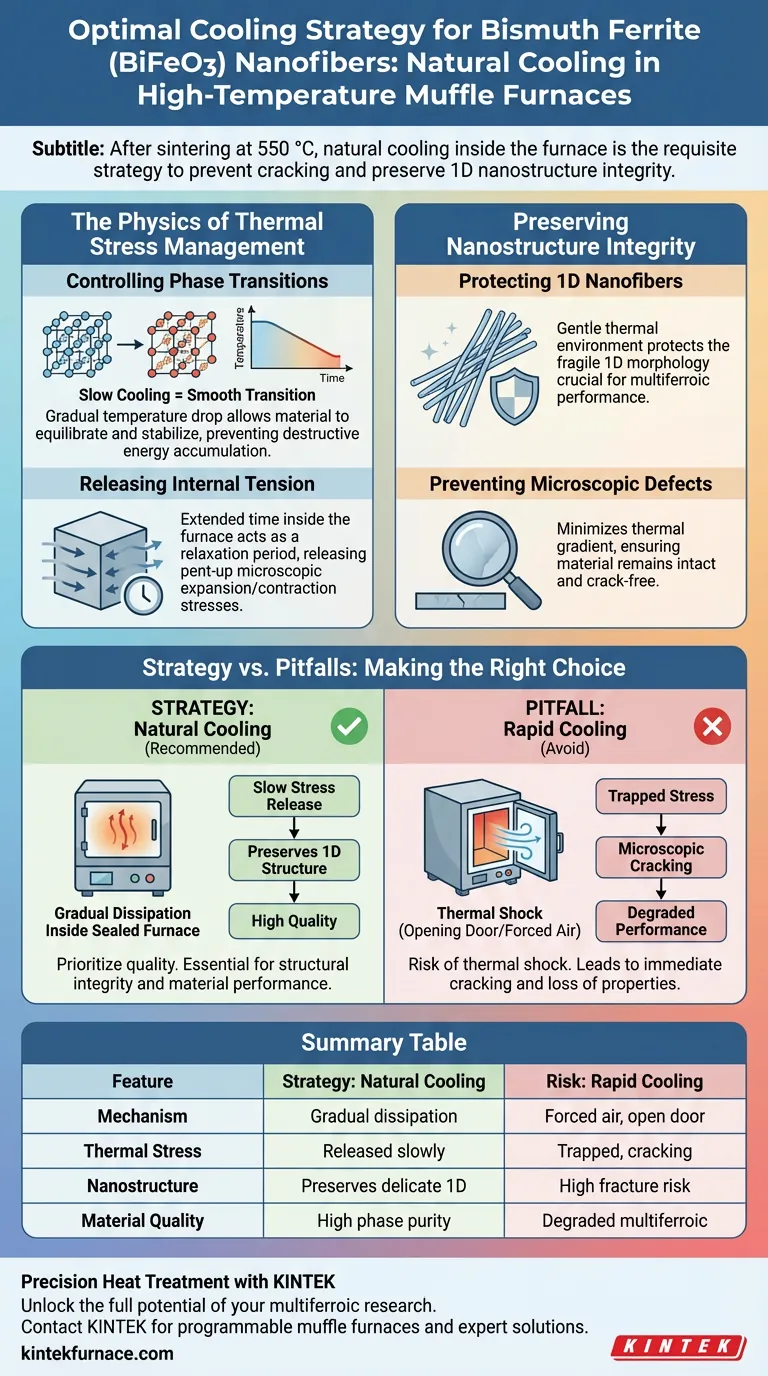
Natural cooling inside the furnace is the requisite strategy for processing bismuth ferrite (BiFeO3) nanofibers following high-temperature calcination. Upon completing the sintering phase (typically at 550 °C), the heating elements should be deactivated, allowing the muffle furnace and the sample to return to room temperature gradually without external interference.
The goal of natural cooling is to facilitate a slow, controlled reduction in temperature. This process is critical for releasing internal thermal stresses generated during phase transitions, thereby preventing microscopic cracking and preserving the structural integrity of the 1D nanofibers.
The Physics of Thermal Stress Management
Controlling Phase Transitions
During the calcination process, bismuth ferrite undergoes significant changes in its internal structure. As the material cools from the sintering temperature of 550 °C, it experiences a phase transition.
If this transition occurs too rapidly, the material does not have time to equilibrate. Natural cooling ensures the temperature drops slowly enough to manage this transition smoothly. This gradual decline allows the material to stabilize without accumulating destructive energy.
Releasing Internal Tension
High-temperature processing inevitably generates internal thermal stresses within the material. These stresses are the result of expansion and contraction differences at the microscopic level.
By keeping the sample inside the furnace, you extend the cooling timeline. This extended duration acts as a relaxation period, effectively releasing these pent-up stresses before the material solidifies completely into its final state.
Preserving Nanostructure Integrity
Protecting 1D Nanofibers
Bismuth ferrite nanofibers possess a delicate 1D (one-dimensional) structure. This morphology is crucial for their performance as a multiferroic material but also makes them physically vulnerable.
Sudden temperature changes can act like a hammer blow to this fragile framework. Natural cooling mitigates this risk by providing a gentle thermal environment.
Preventing Microscopic Defects
The primary danger during the cooling phase is the formation of microscopic cracks. These defects are often invisible to the naked eye but can compromise the entire sample.
When thermal stress exceeds the material's strength, the nanofibers fracture. A natural cooling strategy minimizes the thermal gradient, ensuring the material remains intact and crack-free.
Common Pitfalls to Avoid
The Risk of Thermal Shock
A common error in laboratory settings is opening the furnace door too early to hasten the process. This introduces cold air to the hot sample, causing thermal shock.
Rapid quenching or forced air cooling creates extreme temperature gradients. This almost invariably leads to immediate cracking and the degradation of the bismuth ferrite's multiferroic properties.
Impatience vs. Quality
While natural cooling is time-consuming, it is a non-negotiable trade-off for quality. Prioritizing speed over the cooling rate will negate the benefits gained during the sintering process.
Making the Right Choice for Your Goal
To ensure the successful synthesis of BiFeO3 nanofibers, adhere to the following guidelines:
- If your primary focus is Structural Integrity: strictly follow the natural cooling protocol to prevent the fracturing of delicate 1D nanofibers.
- If your primary focus is Material Performance: allow the slow release of thermal stresses to ensure the final multiferroic properties are not degraded by internal tension.
Success in synthesizing high-quality bismuth ferrite lies not just in the heating, but in the patience exercised during the cooling.
Summary Table:
| Feature | Strategy: Natural Cooling | Risk: Rapid Cooling (Quenching) |
|---|---|---|
| Mechanism | Gradual heat dissipation inside sealed furnace | Opening furnace door or forced air cooling |
| Thermal Stress | Released slowly through relaxation | Trapped, leading to microscopic cracking |
| Nanostructure | Preserves delicate 1D morphology | High risk of fracture and structural collapse |
| Material Quality | High phase purity and integrity | Degraded multiferroic performance |
Precision Heat Treatment for Advanced Nanomaterials
Unlock the full potential of your multiferroic research with KINTEK. Whether you are synthesizing delicate BiFeO3 nanofibers or complex ceramics, our high-temperature muffle furnaces provide the precise thermal control and cooling stability required for sensitive phase transitions.
Backed by expert R&D and manufacturing, KINTEK offers:
- Muffle & Tube Furnaces with programmable cooling rates.
- Vacuum & CVD Systems for high-purity synthesis.
- Customizable Solutions tailored to your unique laboratory requirements.
Ensure your materials remain crack-free and structurally sound. Contact KINTEK today to consult with our specialists about your high-temp lab furnace needs!
Visual Guide
References
- Construction of a 1D/0D/2D BiFeO <sub>3</sub> /Ag/g-C <sub>3</sub> N <sub>4</sub> Z-scheme heterojunction for enhanced visible light photocatalysis of methylene blue. DOI: 10.1039/d5ra04825g
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does an industrial muffle furnace contribute to the thermal treatment of γ-Al2O3 carriers? Optimize Phase Transition
- What is the purpose of using a muffle furnace in incineration? Achieve Pure Ash for Accurate Inorganic Analysis
- What are the standard specifications for Box Furnaces? Key Components for Precision and Efficiency
- Why use an explosion-proof oven for silica aerogels? Essential Safety for High-Temp Ambient Pressure Drying
- What paint industry processes utilize muffle furnaces? Essential for Lab Analysis and Quality Control
- Why are box furnaces important in scientific research? Unlock Precision and Control for Breakthroughs
- What is the function of a box muffle furnace in air annealing? Master Tin Oxide Catalyst Synthesis
- What is the role of convective heat transfer in a box type resistance furnace? Unlock Efficient Heating Dynamics



















